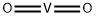Vanadium Dioxide Breaks The Scientific Mold
Sep 29,2019
Imagine a material that can harness excess heat from engines and use it to generate electricity. Imagine that same material could coat windows to trap heat in the winter but let it go in the summer. Imagine that same material working in computer chips, switching itself on and off in less than a ten-billionth of a second.
Vanadium dioxide crystals could potentially make electronic circuits and surface coatings to do all of those things, and researchers from Duke University, Lawrence Berkeley National Laboratory and other institutions are probing the limits of this unique molecule in a study published in the journal Science.
These possibilities all arise from vanadium dioxide’s almost unique ability to conduct electricity but not heat. Olivier Delaire, an associate professor of mechanical engineering and materials science at Duke and corresponding author of the study, joined an international research team to investigate how the electrons in vanadium dioxide move around, since electrons are responsible for conducting both heat and electricity.
In most solids, the ability to conduct electricity is proportional to the ability to conduct heat. In highly conductive materials like metals, the furthest electrons from the nuclei easily flow from one atom to another. That means the electrons are able to flow in a single direction to make electric current, and highly energized electrons that vibrate rapidly are able to knock into nearby electrons and nuclei to transfer that energy in the form of heat. The logic is that if the electrons are free enough to conduct the electric current, they are free enough to transfer heat.
That logic holds true for so many substances that there is a scientific law called the Weidemann-Franz Law that basically says the more a substance can conduct electricity, the more it can conduct heat and vice-versa. Previous studies have shown that vanadium dioxide defies the Weidemann-Franz Law, and while it is not the only substance capable of breaking the law, it is the first that can do so near room temperature. Most other Weidemann-Franz defying materials require super-cold temperatures near absolute zero, making them impractical for everyday use.
That logic holds true for so many substances that there is a scientific law called the Weidemann-Franz Law that basically says the more a substance can conduct electricity, the more it can conduct heat and vice-versa. Previous studies have shown that vanadium dioxide defies the Weidemann-Franz Law, and while it is not the only substance capable of breaking the law, it is the first that can do so near room temperature. Most other Weidemann-Franz defying materials require super-cold temperatures near absolute zero, making them impractical for everyday use.
Vanadium dioxide’s strange properties do not end there, however. It also falls into a class of materials called Mott insulators, which resist heat and current flow at low temperatures, but switch into conductors—which materials scientists call metals—after they warm up. Vanadium dioxide is an insulator until it hits about 150 degrees Fahrenheit, then it turns into an electrical, but not heat, conductor.
Delaire and his colleagues wanted to know how vanadium dioxide is able to behave this way, so they used simulations and x-ray imaging to track the electrons in vanadium dioxide crystals as they changed temperatures.
As predicted, once the vanadium dioxide warmed up to the metal-insulator transition point (MIT) the electrons begin to break away from their home atoms and move more freely. When materials heat up, their electrons start to vibrate. In most solids, those vibrations are completely random: a million particles jiggling at different rates in different directions. When vanadium dioxide’s electrons jiggled, they did so in a synchronized, fluid pattern.
Synchronized vibrations are fine for electrical conduction. A current is like a stream of electrons and as long as the stream keeps flowing in more or less the same direction—it doesn't really matter how the electrons jiggle. A uniform vibration is bad, however, for transferring heat. Delaire says the random motion of the electrons allows them to jump between millions of random configurations, knocking into each other to transfer heat. When the electrons move in rhythm, they don’t have the flexibility to take on all those shapes so heat does not move as effectively through the material.
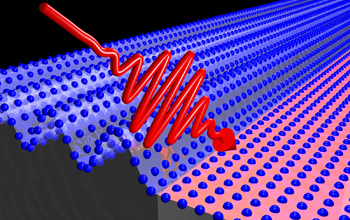
The experiments showed that when vanadium switched from insulator to metal, its electrical conductivity jumped to 1000 times what it was previously. Its thermal conductivity, on the other hand, was about a tenth what the Weidemann-Franz Law predicted it would be.
Now you might be asking yourself, “So what? Vanadium dioxide has this property but how can that possibly be useful?” To answer that, you need to know one final property the researchers discovered. When they doped—the scientific term for adding in—vanadium dioxide crystals with tungsten atoms, they were able to change the metal-insulator transition temperature, and how well the metal phase conducted heat. Being able to precisely control those two properties opens the door to many possibilities.
A coating on a window, for example, could be tuned to conduct heat away from the glass when the temperature rises above 80-degrees Fahrenheit, but could trap heat inside at any temperature below if the metal phase is given high heat conductivity. These materials could trap heat from a warm engine, but harness their conductivity at high temperature to create electricity.
Electrical engineers have been excited by vanadium dioxide’s and other transition metal dioxides’ potential for use in computer transistors. Vanadium dioxide switches from conductor to insulator in one ten-billionth of a second. In a world where faster processing means better technology, being able to turn power on and off 100 times more quickly than the current silicon-based transistors in use today could dramatically improve computing.
Technology like this is still in the future, but for now, the theory that could make it possible is there. The researchers will continue to investigate the limits and uses of vanadium dioxide and whether other compounds can serve the same purpose or improve on those capabilities.
- Related articles
- Related Qustion
Vanadium has incredible properties which increase the strength of alloys containing it, such as rebar which is heavily used in construction. China has just raised their steel rebar limits (raised the bar—no pun intended). Vanadium is also u....
Sep 29,2019Inorganic chemistryCalcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar....
Sep 29,2019Inorganic chemistryVANADIUM(IV) OXIDE
12036-21-4You may like
- Crystal Structure of Aluminum Selenide
Apr 23, 2024
- Crystal Structure and Property of Tin Selenide
Apr 23, 2024
- Crystal Structure of Uranium Carbide
Apr 22, 2024
VANADIUM(IV) OXIDE manufacturers
- VANADIUM(IV) OXIDE
-

- $0.00 / 25KG
- 2023-10-16
- CAS:12036-21-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- VANADIUM(IV) OXIDE
-

- $0.00 / 25KG
- 2023-09-06
- CAS:12036-21-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg