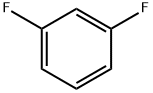
What is 1,3-Difluorobenzene?
Feb 14,2020
1,3-Difluorobenzene (C6H4F2, CAS registry No. 372-18-9) is also named as m-difluorobenzene, which is a clear colorless to yellowish liquid. Its melting point is -59 oC, boiling point is 83 oC and flash point is 2 oC. It is insoluble in water and stable under normal temperatures and pressures. It is toxic and flammable. It will produce toxic fluoride gas by heat. So, the storage environment should be ventilative, low-temperature and dry.
1,3-Difluorobenzene has been extensively used in the synthesis of pharmaceutical and pesticide intermediate. For example, it can be used for the synthesis of fluorinated medicine such as Fluconazole[1], etc. and pesticide (Flucycloxuron, Diflubenzuro, etc.). It can also be used to synthesize liquid crystal materials[2]. Due to the important applications, a series of methods for the preparation of 1,3-difluorobenzene have been reported. There are three methods among which have the application prospects. The first one is Finkelstein Reaction[3]. Fluorine group is introduced by halogen exchange. The route of this method is short. However, due to the consuming of a large amount of fluorinating reagents, high reaction temperature along with long reaction time, and low selectivity, etc., the applications of this method have been limited. Another method is Balz-Schiemann Reaction[4]. The conversion of aryl amines to aryl fluorides is achieved via diazotization and subsequent thermal decomposition of the derived tetrafluoroborates or hexafluorophosphates. it has apparent deficiency such as multiple reaction steps, low yield, and toxic gas boron trifluoride produced, etc.. The last one used for the synthesis of 1,3-difluorobenzene is diazotization and hydro-dediazotization. This method included the diazotization of 2,4-difluoroaniline and hydro-de-diazotization of intermediate diazonium salt. This method can perform at mild conditions with good yield and the diazonium salt is used as a highly reactive intermediate and plays an important role in organic synthesis because of its feature of short reaction time, low reaction temperature, and near complete conversion. However, the raw material used is a bit more expensive than the previous two methods. In general, diazotization reactions are exothermic (ΔH ~ −65 to −150 kJ/mol) and are practically instantaneous even at −10 °C.
Most of the diazonium salts decompose with a heat of decomposition ranging from −160 kJ/mol to −180 kJ/mol which can cause a runaway reaction. So, the accumulation of diazonium salt makes the process unsafe as well as a massive release of nitrogen in hydro-de-diazotization reaction. In consideration of the selectivity and reaction conditions, diazotization and hydro-dediazotization reaction was used for the preparation of 1,3-difluorobenzene. At present, there are two methods to produce 1,3-difluorobenzene via diazotization and hydro-de-diazotization in batch. The first method is stepwise. The aniline reacted with sodium nitrite in the presence of acid, which afforded a diazonium salt. And then the diazonium salt was added into hypophosphorous acid, furnishing the target product. Although the yield is up to 80%, this way has a significant shortcoming of accumulation of highly energetic and potentially explosive diazonium salts, which causes safety incidents easily. So this procedure is potentially hazardous. Another method is producing 1,3-difluorobenzene in one pot. Hypophosphorous acid was firstly added to aniline sulfate, and then sodium nitrite was added slowly. Hydro-de-diazotization reaction occurred in situ as soon as diazonium salt was produced. And the yield could be up to 82% yield. This way seems to be able to solve the safety problem caused by the accumulation of diazonium salt and has concise operation steps. However, it has been found that the rate of hydro-de-diazotization was slower than that of diazotization, which implied that the problem of the accumulation of diazonium salt was still unsolved in batch reactor. The application of continuous-flow technology to the production of 1,3-difluorobenzene, can avoid the cumulative problems of diazonium salt, which is a controllable and practical process for the synthesis of 1,3-difluorobenzene[5].
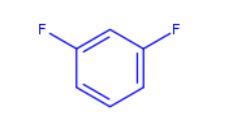
Fig 1. Chemical structure formula of 1,3-difluorobenzene
References
[1]. Jian-xiang., W., Study on the new preparation of fluconazole. Chin J Mod Appl Pharm 2006,(1):35-37., 1, 35-37.
[2]. Pauluth, D.; Tarumi, K., Advanced liquid crystals for television. Journal of Materials Chemistry 2004, 14 (8), 1219-1227.
[3]. Janmanchi, K. M.; Dolbier, J. W. R., Highly Reactive and Regenerable Fluorinating Agent for Oxidative Fluorination of Aromatics. Organic Process Research & Development 2008, 12 (2), 349-354.
[4]. Milner, D. J., Fluoroaromatics from Arylamines, a Convenient One-Pot Conversion Using Nitrosonium Tetrafluoroborate. Synthetic Communications 1992, 22 (1), 73-82.
[5]. Yu, Z.; Lu, G.; Chen, J.; Xie, S.; Su, W., Conversion of 2,4-difluoroaniline to 1,3-difluorobenzene using a continuous-flow reactor. Journal of Flow Chemistry 2018, 8 (2), 51-57.
- Related articles
- Related Qustion
See also
2-Hydroxy-1,4-naphthoquinone (HNQ, C10H6O3, CAS registry No. 83-72-7) is also called Lawsone, which is a white cubic crystal.....
Feb 14,2020Plant extracts4-Cyanobenzyl bromide is an important intermediate for pharmaceutical production. It can be used for the synthesis of a series of piperidine-linked aromatic diimidazolines.....
Feb 14,2020Pharmaceutical intermediates1,3-Difluorobenzene
372-18-9You may like
1,3-Difluorobenzene manufacturers
- 1,3-Difluorobenzene
-
- $100.00 / 1KG
- 2023-12-26
- CAS:372-18-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 1,3-Difluorobenzene
-

- $10.60 / 1KG
- 2023-03-06
- CAS:372-18-9
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 5000kg
- 1,3-Difluorobenzene
-

- $10.00 / 1KG
- 2023-02-18
- CAS:372-18-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt