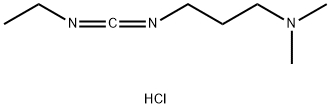
What is 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride?
Jul 9,2020
Overview
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide HCl is Commonly known as EDAC, EDC or EDCI, this carbodiimide HCl salt is used as a coupling reagent in the synthesis of amides and carboxylic esters. EDAC is highly soluble in water and in most organic solvents, it can be employed in liquid and solid-phase and synthesis. The major advantage of EDCI over other carbodiimides such as DCC and DIC is the ease of purification of the product from the water-soluble urea by-product by washing the crude mixture with water or mild acid and extracting in the organic phase.

EDC.HCl, which is commonly regarded as carbodiimide reagents, serves as a very useful tool for the formation of amide bonds (peptide bonds) in an aqueous medium. As one of the carbodiimide reagents, it is also effectively applied in anhydroxydation, polynucleotide synthesis, esterification and lactonization.In addition, EDC.HCl is also widely used as water soluble condensing reagent.
Application
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is being used for the synthesis of amides. EDC.HCl is also used as a coupling agent in the preparation of esters from carboxylic acids using dimethylaminopyridine as the catalyst. It is water-soluble carbodiimide, widely used for peptide coupling.N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride has been used modify the cell surface of Escherichia coli to covalently couple substances. It has also been used as an activator to modify microfluidic chips to capture Escherichia coli.
Formation of Peptide Bonds Using EDC·HCl /HOBt
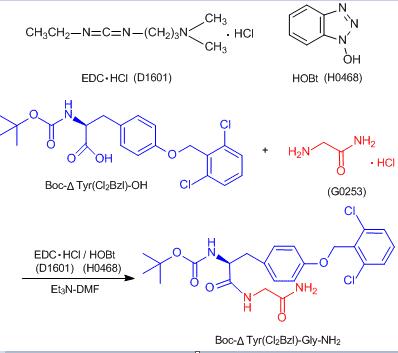
Typical Procedure:
To a solution of Boc-ΔTyr(Cl2Bzl)-OH (307 mg, 0.7 mmol), glycinamide hydrochloride (77.4 mg, 0.7 mmol) and Et3N (0.1 mL, 0.7 mmol) in DMF (7 mL) is added EDC·HCl (148 mg. 0.77 mmol) and HOBt (129 mg, 0.84 mmol) at -10°C. The reaction mixture is stirred for 2 h at 0°C. and overnight at room temperature. After the solution is evaporated, the residual is dissolved in EtOAc. The solution is washed with 4% NaHCO3, 10% citric acid and water. The solution is dried and evaporated. The purification is carried out by silica gel column chromatography to give Boc-ΔTyr(Cl2Bzl)-Gly-NH2 (317 mg, 85% yield).
Water-soluble Condensation Reagent
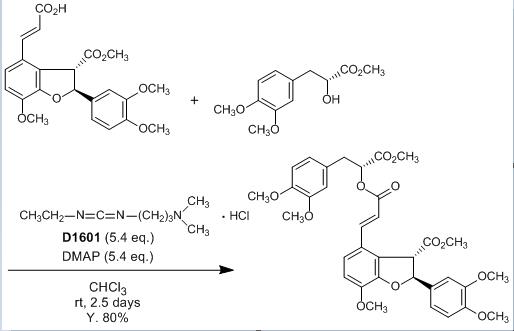
The main applications of EDAC are in peptide synthesis, Steglich esterification reactions in presence of catalytic DMAP, immunoconjugate synthesis, synthesis of sulfo-NHS esters and coupling of biomolecules onto solid supports.
Biochem/physiol Actions
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide is a water soluble condensing reagent. EDAC is generally utilized as a carboxyl activating agent for amide bonding with primary amines. Additionally, it reacts with phosphate groups. It has been utilized in peptide synthesis, crosslinking proteins to nucleic acids as well as preparation of immunoconjugates.
Water soluble condensing reagent. EDAC is generally utilized as a carboxyl activating agent for amide bonding with primary amines. In addition, it will react with phosphate groups. EDAC has been used in peptide synthesis; crosslinking proteins to nucleic acids; and preparation of immunoconjugates as examples. Typically, EDAC is utilized in the pH range 4.0-6.0 without buffers. In particular, amine and carboxylate buffers should be avoided.
Purification Methods
It is an excellent H2O-soluble peptide coupling reagent. It is purified by dissolving (ca 1g) in CH2Cl2 (10mL) at room temperature and then add dry Et2O (~110mL) dropwise and the crystals that separate are collected, washed with dry Et2O, recrystallised from CH2Cl2/Et2O and dried in a vacuum over P2O5. It is important to work in a dry atmosphere or work rapidly and then dry the solid as soon as possible. The material is moderately hygroscopic, but once it becomes wet it reacts slowly with H2O. Store it away from moisture at -20o to slow down the hydrolysis process. The free base has b 47-48o/0.27mm, 53-54o/0.6mm, n 1.4582. The methiodide is recrystallised from CHCl3/EtOAc, the crystals are filtered off, washed with dry Et2O, recrystallised from CHCl3/Et2O, and dried in vacuo over P2O5, m 93-95o, 94-95o. [Sheehan et al. J Am Chem Soc 87 2492 1965, Sheehan & Cruickshank Org Synth Coll Vol V 555 1973.] § A polymer bound version is commercially available.
Safety and Handling
Hazard Category
Serious eye damage/eye irritation Category 2
Respiratory sensitiser Category 1
Skin corrosion/irritation Category 2
Specific target organ toxicity Category 3
GHS H Statement
Harmful if swallowed.
Causes skin irritation.
May cause an allergic skin reaction.
Causes serious eye irritation.
GHS P Statement
Wear protective gloves/protective clothing/eye protection/face protection.
IF SWALLOWED: rinse mouth.
Do NOT induce vomiting.
IF ON SKIN: Wash with plenty of soap and water.
If eye irritation persists: Get medical
- Related articles
- Related Qustion
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride: Synthesis method and uses Apr 16, 2024
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is a white crystalline powder that easily absorbs moisture.
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride: Application, Storage, Safety Apr 26, 2023
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, also known as EDC·HCl, is a popular carbodiimide coupling reagent used in bioconjugation chemistry.
Hyaluronic acid or hyaluronan, is actually a carbohydrate molecule that naturally occurs in our bodies. To answer the question you are asking yourself, yes, it’s already in our skin!....
Jul 8,2020Biochemical EngineeringBoron nitride is a material in which the extra electron of nitrogen (with respect to carbon) enables it to form structures that are isoelectronic with carbon allotropes.Boronnitride is manufactured by heatingboron oxide to 800°C.....
Jul 14,2020Inorganic saltsYou may like
- What you need to know about ceramides?
Apr 17, 2024
- A formaldehyde releasing agent: Quaternium-15
Jan 12, 2024
- Oleamide: Physiological effects
Jul 18, 2023
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride manufacturers
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
-

- $0.00 / 25kg
- 2024-04-23
- CAS:25952-53-8
- Min. Order: 100g
- Purity: 99%
- Supply Ability: 100mt
- EDC HCL
-

- $9.00 / 10g
- 2024-04-22
- CAS:25952-53-8
- Min. Order: 10g
- Purity: min99%
- Supply Ability: 10 tons
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
-

- $50.00 / 1kg
- 2024-04-20
- CAS:25952-53-8
- Min. Order: 1kg
- Purity: 99.10%
- Supply Ability: 5000kg