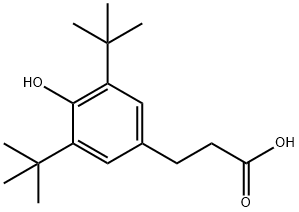
What is 3-(3,5-Di-tert-butyl-4-hydroxyphenyl)propionic acid?
Apr 1,2020
Chemical Properties
3-(3, 5-tert-butyl-4-hydroxyphenyl) propionic acid with a molecular formula of C17H26O3 , can irritate the eyes, airways and skin.
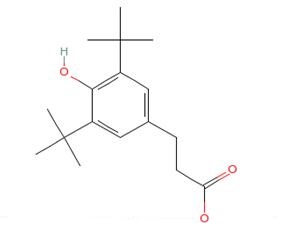
Fig 1 Sructure of 3-(3, 5-tert-butyl-4-hydroxyphenyl) propionic acid
Application
One important application of 3-(3, 5-tert-butyl-4-hydroxyphenyl) propionic acid is in the synthesis of antioxidant. Antioxidants are a component of the majority of known polymerous stuffs and consumer properties of products depend on their efficacy. Antioxidants of phenolic structure are additives to polymers because of high performance to protect polymers from thermal-oxidative degradation processes. From this class of stabilizers the most widespread are derivatives of a methyl ester 3-(3’,5’-di-tert.buyil-4’-hylroxyphenyl)-propionic acid, productions which are based on ester exchanges polyatomic alcohols. To the most significant is pentaeritril-tetrakis-3-(3’,5’-di-tert.buyil-4’- hylroxyphenyl)-propionate. From the beginning of works on synthesis of derivatives of a methyl ester 3-(3’,5’-di-tert.buyil-4’-hylroxyphenyl)- propionic acid on the present are executed a series of basic researches on catalysis and technology. These workings out can appear perspective for improvement of technology on the basis of building of continuous process of reception of a methyl ester 3-(3’,5’-di-tert.buyil-4’-hylroxyphenyl)- propionic acid. In the conditions of catalysis with use of potassium or sodium hydroxides a methyl ester 3-(3’,5’-di-tert.buyil-4’-hylroxyphenyl)- propionic acid is formed with yields to 98%. That allows using a main product further in ester exchange by pentaerythritol. In this technology power-intensive stages of allocation by fractionation in vacuo and stages of use of solvents that can render positive influences on the cost price and an ecological situation by production of the specified antioxidant are excluded.[1] And in the present work 3-(3,5-di-tert-butyl-4-hydroxy-phenyl)-propionic acid can be used as antioxidant group to modify esters. This kind of esters is useful in many fields, such as lubricating oil and cutting compound, because of two important functions (antioxidant and lubricant) in the same compound. Furthermore, the esters were characterised with Fourier transform infrared spectroscopy (FTIR) and thermogravimetric (TG) analysis.
The analytical results of FTIR demonstrates the changes of alcoholic hydroxyl and carboxyl and suggests that this synthesis was feasible. The TG analysis and rotating pressure vessel method show that this kind of esters has excellent oxidative stability. The physical properties of lubricating base oil, such as viscosity, viscosity index, pour point and flash point, suggested that this kind of esters can be used as lubricating base oil.[2]
In addition, unsaturated esters of 3,5-di-tert-butyl-4-hydroxybenzoic acid and 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid were successfully synthesized and characterized using FT-IR and NMR spectroscopy. The effect of chain length and phenolic group is investigated on efficiency of antioxidants. It is demonstrated that the esters of 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid exhibit significantly longer induction time. The results of radical grafting reaction shows synthesized antioxidants can be successfully grafted onto polymer chains and the phenolic moiety is functional after extraction process, while pure and commercially stabilized samples are degraded instantaneously. Also, different initiator systems are utilized to enhance the extent of grafting. Among MEK, DCP, and DHBP peroxides, DHBP can be more effective in increasing the antioxidant grafted onto polymer. In addition, possibility of rising in graft content is investigated in presence of redox initiator. Using this approach, polymerbound antioxidant with prolonged thermal stability can be achieved.[3]
Safety
The safety of related compounds also attracted attention. For example, the toxicity of 3-(3,5-di-tert-butyl-4- hydroxyphenyl)propanoic acid, esters with C13-C15 branched and linear alcohols has been tested by EFSA. And this scientific opinion of EFSA deals with the risk assessment of the additive, 3-(3,5-di-tert-butyl-4- hydroxyphenyl)propanoic acid, esters with C13-C15 branched and linear alcohols, CAS No. 171090- 93-0, REF. No. 47060 for which the CEF Panel concluded that there is no safety concern for the consumer if the substance is used only in polyolefins in contact with foods other than fatty/highalcoholic and dairy products and its migration does not exceed 0.05 mg/kg food.[4]
References
[1] ZAIKOV, G., VOLODKIN, À., LOMAKIN, S., & KUBICA, S. ON RECEPTION OF THE METHYL ESTER 3-(3’, 5’-DI-TRET. BUTYL-4’-HYDROXYPHENYL)-PROPIONIC ACID.
[2] Zhang, L., Cai, G., Wang, Y., & Eli, W. (2012). Synthesis and characterisation of antioxidant-modified esters of dipentaerythritol as lubricating base oil. Lubrication Science, 25(5), 329–337. doi:10.1002/ls.1183
[3] Manteghi, A., Ahmadi, S., & Arabi, H. (2016). Polyolefin elastomer grafted unsaturated hindered phenol esters: synthesis and antioxidant behavior. Designed Monomers and Polymers, 19(6), 569–576. doi:10.1080/15685551.2016.1187442
[4] EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF). (2011). Scientific Opinion on the safety evaluation of the substance, 3‐(3, 5‐di‐tert‐butyl‐4‐hydroxyphenyl) propanoic acid, esters with C13‐C15 branched and linear alcohols, CAS No. 171090‐93‐0, for use in food contact materials. EFSA Journal, 9(2), 1998.
- Related articles
- Related Qustion
Methanesulfonyl chloride is also called methyl sulfonyl chloride, mesyl chloride, methanesulfonic acid chloride. It is a colorless transparent liquid, soluble in ethanol and ether, slightly soluble in water, and corrosive.....
Mar 30,2020Organic reagentsAcetophenone is a colorless crystal, or light yellow oily liquid, and has many unique chemical properties. It has been reported previously that the hydrolysis of acetophenone diethyl ketals is not subject to general acid catalysis.....
Apr 1,2020Organic Raw MaterialYou may like
3-(3,5-Di-tert-butyl-4-hydroxyphenyl)propionic acid manufacturers
- 3-(3,5-Di-tert-butyl-4-hydroxyphenyl)propionic acid
-

- $200.00 / 20KG
- 2024-04-16
- CAS:20170-32-5
- Min. Order: 1000KG
- Purity: 98
- Supply Ability: 1000m.t per year
- 3-(3,5-Di-tert-butyl-4-hydroxyphenyl)propionic acid
-

- $1.00 / 1KG
- 2019-07-14
- CAS:20170-32-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 25kg