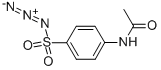
What is 4-Acetamidobenzenesulfonyl azide?
Feb 18,2020
4-Acetamidobenzenesulfonyl azide is an important organic intermediate (building block) to synthetize substituted amidobenzene products.
The following example is about its application of treated catalysts in hydrogenation reactions [1].

A mixture of substrate (0.2 mmol) and Pd/Al2O3Si (6.4 mg, 1 mol%) in ethanol (2 mL) was stirred under hydrogen atmosphere (H2 balloon pressure) at room temperature. The progress of reaction was monitored by TLC analysis or GC-MS. After the reaction was completed, the reaction mixture was filtered, and the catalyst was washed with ethanol. The solvent in filtrate was removed by vacuum to give the crude product. The purified product was obtained by column chromatography or recrystallization. After each cycle, the palladium residue in the reaction mixture solvent was determined by ICP-OES.
The following example is about its application on the synthesis of 5-((10S)-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl)-1-(ethyloxy)-1,3-dioxo-2-pentanediazonium [2].
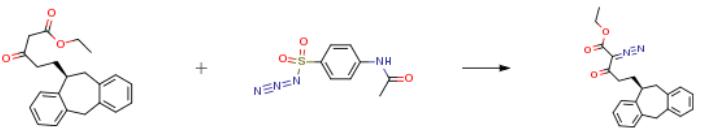
Ethyl 5-((10S)-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl)-3-oxopentanoate (433 g, 1.29 mol) was dissolved in acetonitrile (8 L). TEA (197 ml, 1.42 mol) was added and finally 4-(acetylamino)benzenesulfonyl azide (340 g, 1.42 mol) portion wise at rt. A clear solution was obtained. After 30 min a strong precipitation occurred. After 16 hours the solfonamide was filtered off and the solution was diluted with ethyl ether (4L) and washed with NH4Cl sat solution (2×5L) and water (5L) left to stir 30 min each time. The organic layers were dried (Na2SO4) and concentrated in vacuum. The crude product was purified by a pad of silica gel (2 Kg) eluting with a gradient of ethyl acetate in cyclohexane (0 to 10 percent) to afford the final compound (438 g, 94 percent yield).
The following example is about its application on the synthesis of inhibitors of Hepatitis C Virus RNA-dependent RNA polymerase [3].
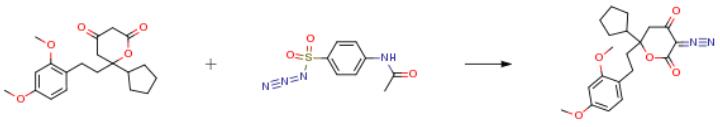
To a solution of 6-Cyclopentyl-6- [2- (2, 4-dimethoxy-phenyl) -ethyl]-dihydro- pyran-2,4-dione (0.5g, 1.44 mmol) and sodium hydrogen phoshate (monobasic) (0.26g, 2.16 mmol), dissolved in 6 ml of DMF at room temperature, under Argon, was added 4-acetamido benzenesulfonyl azide (0.52g, 2.16 mmol). The resulting mixture was stirred for 4 hours, during which time starting material disappearance was monitored via TLC. The reaction was quenched at this time by adding sodium hydrogen phosphate (dibasic) (40 ml). The resulting mixture was extracted with EtOAc (3 x 30 ml), water (20 ml), and dried over anhydrous Na2SO4. The result material was purified by column chromatography using Hexanes: EtOAc (1: 1) yielding the product in quantitative yield.
The following example is about its application on the synthesis of ethyl diazo(diethoxyphosphoryl)acetate [4].
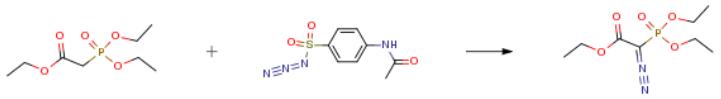
To a suspension of 60% sodium hydride in mineral oil (0.6 g, 15 mmol) in THF (100 mL) was added dropwise ethyl (diethoxyphosphoryl)acetate 12 (2.2 g, 10 mmol) at 0 °C, and the resulting mixture was stirred at 0 °C for 0.5 h. To the reaction mixture was added 4-acetamidobenzenesulfonyl azide (2.5 g, 10.5 mmol) at 0 °C, and the mixture was stirred at room temperature for 1 h. The reaction mixture was diluted with EtOAc/water (1:1, 200 mL). After acidified to pH 2 with 1 M HCl, the aqueous layer was extracted with EtOAc (2 × 50 mL). The combined organic layers were dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography (silica gel, eluted with 0-100% EtOAc in n-hexane) to give the product (2.5 g, quantitative) as colorless oil.
References
1.Zhou M, Li T, Xu B. Easy-handling and low-leaching heterogeneous palladium and platinum catalysts via coating with a silicone elastomer[J]. Tetrahedron Letters, 2019, 60(14):948 - 952
2.Gianotti M, Andreotti D, Casotto D, Mattioli M, Mingardi A, Pavone F, Profeta R, Valente F. Asymmetric route to spirotetracyclic (1S)-5′,11′-dihydro-3H- spiro[cyclopentane-1,10′-dibenzo[a,d]cyclohepten]-3-one derivatives[J]. Tetrahedron Letters, 2011, 52(2):329 - 331
3.Pfizer Inc. Inhibitors of Hepatitis C Virus RNA-dependent RNA polymerase, and compositions and treatments using the same. WO2003/95441[P], 2003, A1, Page/Page column 145
Hirayama T, Okaniwa M, Imada T, Ohashi A, Ohori M, Iwai K, Mori K, Tanaka T, Ishikawa T. Synthetic studies of centromere-associated protein-E (CENP-E) inhibitors: 1.Exploration of fused bicyclic core scaffolds using electrostatic potential map[J]. Bioorganic and Medicinal Chemistry, 2013, 21(17):5488 - 5502
- Related articles
- Related Qustion
See also
As an NNN-tridentate ligand, the 2,2′:6′,2″-terpyridine plays an important role in coordination chemistry. With three coordination sites and low LUMO, terpyridine and its derivatives are one of the typical Pincer ligand.....
Feb 18,2020Pyridine CompoundTributyltin hydride (TBTH) is as a reagent for the reductive ionic 1,5-cyclization of 3-azamuconoates (3-azahexa-2,4-dienedioates). Agafonova reported that a novel 1,5-exo-trig-cyclization of 5-chloro-3-azamuconoates can be used as a base.....
Feb 18,2020Organometallic compounds4-Acetamidobenzenesulfonyl azide
2158-14-7You may like
4-Acetamidobenzenesulfonyl azide manufacturers
- 4-Acetamidobenzenesulfonyl azide
-
- $100.00 / 1KG
- 2023-12-26
- CAS:2158-14-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 4-Acetamidobenzenesulfonylazide
-
- $0.00 / 1KG
- 2022-01-15
- CAS:2158-14-7
- Min. Order: 1KG
- Purity: 98.2%
- Supply Ability: 100 tons
- 4-Acetamidobenzenesulfonyl azide
-

- $0.10 / 1KG
- 2020-01-08
- CAS:2158-14-7
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 1000 tons