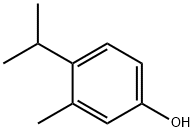
What is 4-Isopropyl-3-methylphenol?
Jan 13,2020
Isopropylmethylphenols are widely used for products such as medicaments, quasi-drugs and cosmetics, as their agents such as an antibacterial agent, a bactericidal agent, and an antiseptic. Isomers exist in an isopropylmethylphenol; among them, a 4-isopropyl-3-methylphenol has characteristics such that its antibacterial and bactericidal activities are strong, as well as its toxicity against skin irritation is low, and it is colorless and odorless. In fact, 4-isopropyl-3-methylphenol is a synthetic analog of the natural products thymol (2-isopropyl-5-methylphenol) and carvacrol (5-isopropyl-2-methylphenol).
4-Isopropyl-3-methylphenol can be produced by the isopropylation of m-cresol via different catalysts ((e.g., calcium oxide, a metal sulfate and γ-alumina, a catalyst solution including a zinc bromide, hydrogen bromide and water) with respective reaction conditions. There are various isomers produced during the reaction such as 4-isopropyl-3-methylphenol, 2-isopropyl 3-methylphenol, 5-isopropyl 3-methylphenol and thymol (6-isopropyl-3-methylphenol) along with isopropyl-3-methyl-benzylether, dialkylated and trialkylated products. Hence selectivity plays an important role in synthesizing.1 Then these mixture undergo refinement controls such as isomerization, distillation, extracting, and crystallization to get the targeted product.
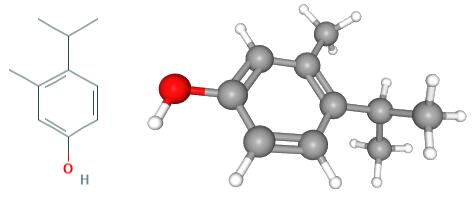
Fig 1. Chemical structure of 4-isopropyl-3-methylphenol isopropylation of m-cresol
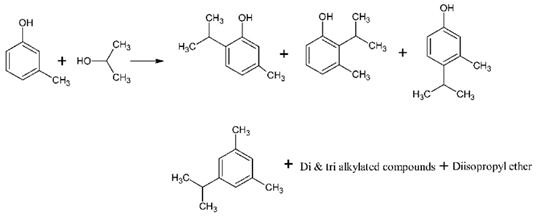
Scheme 1. Production of 4-isopropyl-3-methylphenol
The applications of 4-isopropyl-3-methylphenol have been reported as follows:
- As an antimicrobial preservative: as with thymol and carvacrol, 4-isopropyl-3-methylphenol has also been developed as an antimicrobial preservative, where its color/odor-neutral characteristic is more appealing to consumer perception. Like the natural analog thymol, 4-isopropyl-3-methylphenol might also be involved in cellular ion (which links to xenobiotic detoxification) and redox homeostasis. Phenolics function as deleterious redox cyclers against microbial cells, and thus prevent microbial growth by disrupting cellular redox homeostasis. Consequently, fungi having deficiency in vacuolar/antioxidant systems, viz., abnormality in xenobiotic detoxification and antioxidation, would be more susceptible to 4-isopropyl-3-methylphenol.2
- As one of the phenolic metabolites: The mixture of 4-isopropyl-3-methylphenol and thymol, together with 3,4-O-dicaffeoylquinic acid methyl ester and two dicaffeoylquinic acid derivatives were reported responsible for analgesic and anti-inflammatory effects. In addition, such investigations suggested that the anti-inflammatory activity of these metabolites is related to their effects as radical scavengers.3
- As an acaricidal agent: In the fumigant toxicity bioassay, 4-isopropyl-3-methylphenol (0.78 μg/cm2) was more effective than the commercial acaricide benzyl benzoate (10.78 μg/cm2) against D. farina. In addition, in the direct-contact toxicity bioassay, 4-isopropyl-3-methylphenol (0.56 μg/cm2) was more toxic than benzyl benzoate (7.73 μg/cm2) against D. farinae. Similar effects were found in the case against D. pteronyssinus. In structure–activity relationships, acaricidal activities could be related to the introduction of isopropyl functional group onto the 3-methylphenol skeleton. These results indicate that naturally occurring 3-methylphenol and its derivatives are potential house dust mite control agents.4
- As a non linear optics: transparent single crystals of different phenols are grown from the melt or from saturated solution in various solvents. Their crystal structure determination are studied by x-ray or neutron diffraction methods. Among them several crystals are used to measure physical properties, and the crystallization, the crystal structure and the nonlinear optical properties of 4-isopropyl-3-methylphenol can be carefully studied, whose large single crystals can be obtained (10 cm3 in volume).5
References
1. Malkar, R. S.; Yadav, G. D., Selectivity Engineering in Synthesis of Thymol Using Sulfated ZrO2–TiO2. Industrial & Engineering Chemistry Research 2017, 56 (30), 8437-8447.
2. Kim, J. H.; Hart-Cooper, W.; Chan, K. L.; Cheng, L. W.; Orts, W. J.; Johnson, K., Antifungal efficacy of octylgallate and 4-isopropyl-3-methylphenol for control of Aspergillus. Microbiology Discovery 2016, 4 (1), 2.
3. Mijangos-Ramos, I. F.; Zapata-Estrella, H. E.; Ruiz-Vargas, J. A.; Escalante-Erosa, F.; Gómez-Ojeda, N.; García-Sosa, K.; Cechinel-Filho, V.; Meira-Quintão, N. L.; Peña-Rodríguez, L. M., Bioactive dicaffeoylquinic acid derivatives from the root extract of Calea urticifolia. Revista Brasileira de Farmacognosia 2018, 28 (3), 339-343.
4. Jeon, J.-H.; Yang, J.-Y.; Chung, N.; Lee, H.-S., Contact and Fumigant Toxicities of 3-Methylphenol Isolated from Ostericum koreanum and Its Derivatives against House Dust Mites. J. Agric. Food. Chem. 2012, 60 (50), 12349-12354.
5.https://pubchem.ncbi.nlm.nih.gov/compound/18597
6.https://www.chemspider.com/Chemical-Structure.17564.html?rid=809d6800-b966-48b1-b461-2217ecc2c5b2
- Related articles
- Related Qustion
2-hydroxyethyl methacrylate (HEMA) is a commercially important and widely used monomer. It is commonly prepared in a one-step reaction from methyl methacrylate or methacrylic acid.....
Jan 13,2020Organic SolventsIn 4-Pyridinepropanol molecule, the unpaired electrons of nitrogen are not involved in the resonance structure of the pyridine aromatic ring, i.e. perpendicular to the aromatic plain.....
Jan 13,2020Heterocyclic compounds4-ISOPROPYL-3-METHYLPHENOL
3228-02-2You may like
- Pyriofenone: Synthesis and Application
Feb 5, 2024
- How is Picarbutrazox synthesised and what does it do?
Feb 5, 2024
- How is Tolprocarb synthesised?
Feb 4, 2024
4-ISOPROPYL-3-METHYLPHENOL manufacturers
- 4-isopropyl-3-methylphenol (IPMP)
-

- $0.00 / 25Kg/Drum
- 2024-04-23
- CAS:3228-02-2
- Min. Order: 100g
- Purity: 99%
- Supply Ability: 500mt/year
- 4-ISOPROPYL-3-METHYLPHENOL
-
- $8.00 / 1KG
- 2024-04-09
- CAS:3228-02-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 4-ISOPROPYL-3-METHYLPHENOL
-

- $20.00 / 1kg
- 2023-11-04
- CAS:3228-02-2
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20 tons