What is Binimetinib?
Feb 21,2020
Binimetinib, also known as Mektovi, is a potent and selective oral mitogen-activated protein kinase 1/2 (MEK 1/2) inhibitor with potential antineoplastic activity[1].
Description
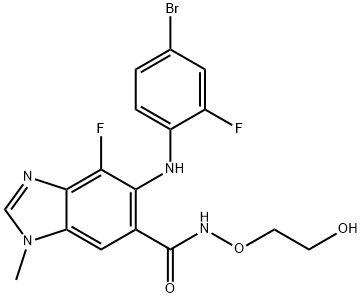
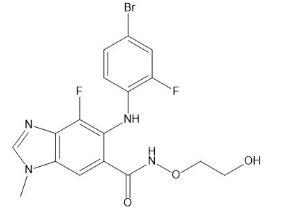
Binimetinib is a kinase inhibitor. The chemical name is 5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2hydroxyethoxy)- 1-methyl-1H-benzimidazole-6-carboxamide. The molecular formula is C17H15BrF2N4O3 and the molecular weight is 441.2 daltons [2].
Binimetinib, noncompetitive with ATP, binds to and inhibits the activity of MEK1/2. Inhibition of MEK1/2 prevents the activation of MEK1/2 dependent effector proteins and transcription factors, which may result in the inhibition of growth factor-mediated cell signaling. This may eventually lead to an inhibition of tumor cell proliferation and an inhibition in production of various inflammatory cytokines including interleukin-1, -6 and tumor necrosis factor. MEK1/2 are dual-specificity threonine/tyrosine kinases that play key roles in the activation of the RAS/RAF/MEK/ERK pathway and are often upregulated in a variety of tumor cell types [3].
Mechanism of Action
Binimetinib is a reversible inhibitor of mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. In vitro, binimetinib inhibited extracellular signal-related kinase (ERK) phosphorylation in cellfree assays as well as viability and MEK-dependent phosphorylation of BRAF-mutant human melanoma cell lines. Binimetinib also inhibited in vivo ERK phosphorylation and tumor growth in BRAF-mutant murine xenograft models.
Binimetinib and encorafenib target two different kinases in the RAS/RAF/MEK/ERK pathway. Compared to either drug alone, coadministration of encorafenib and binimetinib resulted in greater anti-proliferative activity in vitro in BRAF mutation-positive cell lines and greater anti-tumor activity with respect to tumor growth inhibition in BRAF V600E mutant human melanoma xenograft studies in mice. Additionally, the combination of binimetinib and encorafenib delayed the emergence of resistance in BRAF V600E mutant human melanoma xenografts in mice compared to either drug alone.
Pharmacokinetics
The pharmacokinetics of binimetinib was studied in healthy subjects and patients with solid tumors. After twice-daily dosing, the accumulation is 1.5-fold and the coefficient of variation (CV%) of the area under the concentration-time curve (AUC) is < 40% at steady state. The systemic exposure of binimetinib is approximately dose proportional.
Metabolism
The primary metabolic pathway is glucuronidation with UGT1A1 contributing up to 61% of the binimetinib metabolism. Other pathways of binimetinib metabolism include N-dealkylation, amide hydrolysis, and loss of ethane-diol from the side chain. The active metabolite M3 produced by CYP1A2 and CYP2C19 represents 8.6% of the binimetinib exposure. Following a single oral dose of 45 mg radiolabeled binimetinib, approximately 60% of the circulating radioactivity AUC in plasma was attributable to binimetinib [4].
Side Effects
Important things to remember about the side effects of binimetinib (when administered with encorafenib):
- Most people will not experience all of the binimetinib side effects listed
- Side effects are often predictable in terms of their onset, duration, and severity.
- Side effects are almost always reversible and will go away after treatment is complete.
- Side effects may be quite manageable. There are many options to minimize or prevent the side effects of binimetinib.
- The following side effects are common (occurring in greater than 30%) for patients taking binimetinib with encorafenib:
- Fatigue
- Nausea and vomiting
- Diarrhea
- Increased serum creatinine (monitor with other nephrotoxic medications [5]
References
[1] https://www.drugbank.ca/drugs/DB11967
[2] https://www.rxlist.com/mektovi-drug.htm#side_effects
[3] https://pubchem.ncbi.nlm.nih.gov/compound/Binimetinib
[4] https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210498lbl.pdf
[5] https://chemocare.com/chemotherapy/drug-info/binimetinib.aspx
- Related articles
- Related Qustion
- The synthesis method of Binimetinib Dec 27, 2023
Binimetinib is a non-ATP competitive mitogen-activated protein kinase 1/2 (MEK1/2) inhibitor discovered by Array BioPharma.
Isoparaffin l, synthesis grade, also called White Spirit Type 3 (EINECS number: 265-150-3; CAS number:64742-48-9) or hydrotreated heavy naphtha (petroleum), is a complex combination of hydrocarbons.....
Feb 21,2020Organic Raw MaterialN,N-Dimethylformamide, or dimethylformamide is an organic compound with the formula (CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate).....
Feb 21,2020AmidesBinimetinib
606143-89-9You may like
- Binimetinib
-

- $0.00/ kg
- 2023-12-24
- CAS:606143-89-9
- Min. Order: 1kg
- Purity: 99%, Single impurity<0.1
- Supply Ability: 1 ton
- Binimetinib
-

- $15.00 / 1KG
- 2021-07-13
- CAS:606143-89-9
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Binimetinib
-

- $15.00 / 1KG
- 2021-07-10
- CAS:606143-89-9
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton