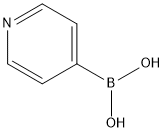
What is Pyridine-4-boronic acid?
Feb 13,2020
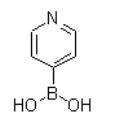
References
- Related articles
- Related Qustion
- Pyridine-4-boronic acid: properties, applications and safety Sep 26, 2023
Pyridine-4-boronic acid is a versatile compound with unique properties, used in various organic chemistry applications and drug discovery.
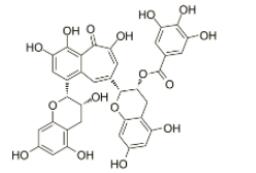
Theaflavin-3-gallate, a black tea theaflavin monomer, formed in the oxidation of epicatechin (EC) and epigallocatechin gallate (EGCG) at the ratio of 1:1, is regarded as the biologically important active component of black tea.....
Feb 13,2020Natural Products7,9-Ditert-butyl-1-oxaspiro[4.5]deca-6,9-diene-2,8-dione (C17H24O3, CAS registry No. 82304-66-3) is an oxaspiro compound that is 1-oxaspiro[4.5]deca-6,9-diene-2,8-dione carrying two additional tert-butyl substituents at positions 7 and 9.....
Feb 13,2020Natural ProductsPyridine-4-boronic acid
1692-15-5You may like
- Tert-Butyldimethylsilyl chloride: Uses and hazard
Apr 24, 2024
- What kind of substance is triethylenediamine?
Apr 24, 2024
- The uses of Acetyl chloride
Apr 24, 2024
Pyridine-4-boronic acid manufacturers
- Pyridine-4-boronic acid
-

- $0.00 / 1kg
- 2023-11-27
- CAS:1692-15-5
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 30000 Kg
- 3-Pyridylboronic acid
-

- $35.00/ kg
- 2023-10-28
- CAS:1692-15-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 Ton
- Pyridine-4-boronic acid
-

- $0.00 / 25Kg/Drum
- 2023-01-31
- CAS:1692-15-5
- Min. Order: 1Kg/Drum
- Purity: 99%
- Supply Ability: 5000KG