100189-16-0
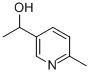 100189-16-0 结构式
100189-16-0 结构式
基本信息
Α,6-二甲基-3-吡啶甲醇
1-(6-甲基-3-吡啶基)乙醇
5-(1-羟基乙基)-2-甲基吡啶
a,6-diMethyl-3-PyridineMethanol
1-(6-Methyl-3-pyridinyl)ethanol
α,6-DiMethyl-3-pyridineMethanol
3-Pyridinemethanol, α,6-dimethyl-
3-Pyridinemethanol, a,6-dimethyl-
5-(1-Hydroxyethyl)-2-methylpyridine
3-(1-Hydroxyethyl)-6-Methylpyridine
dl-2-Methyl-5-[1-hydroxyethyl]pyridine
3-Pyridinemethanol,alpha,6-dimethyl-(6CI,9CI)
物理化学性质
制备方法
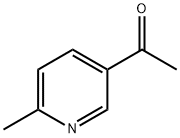
36357-38-7

56019-64-8
以1-(6-甲基吡啶-3-基)乙酮为原料合成6-甲基-3-(1-羟乙基)吡啶的一般步骤:在30℃条件下,将硼氢化钠(2.3g,0.06mol)分批加入至1-(6-甲基吡啶-3-基)乙酮(16.4g,0.121mol)的乙醇(160ml)溶液中,保持温度恒定并持续搅拌反应混合物。反应1小时后,用饱和碳酸氢钠溶液(2×200ml)稀释反应混合物,随后用二氯甲烷(2×500ml)进行萃取。合并有机相,经无水硫酸钠干燥后浓缩,得到浅黄色油状物。该粗产物通过快速柱色谱法(洗脱剂:5%甲醇/二氯甲烷)纯化,得到6-甲基-3-(1-羟乙基)吡啶(17.0g,两步收率93%),为淡黄色油状物。ES-MS [M + 1]+:138.1。1H NMR(400MHz,CDCl3):δ 8.35(d,J = 2.0Hz,1H),7.63(dd,J = 8.0,2.4Hz,1H),7.12(d,J = 8.0 Hz,1H),4.89(q,J = 6.5 Hz,1H),3.30(brs,1H),2.50(s,3H),1.48(d,J = 6.5 Hz,3H)。
参考文献:
[1] Journal of Medicinal Chemistry, 1996, vol. 39, # 26, p. 5053 - 5063
[2] Chemical and Pharmaceutical Bulletin, 1995, vol. 43, # 12, p. 2168 - 2172
[3] Chemische Berichte, 1947, vol. 80, p. 502,505
[4] Patent: WO2009/80637, 2009, A1. Location in patent: Page/Page column 32
[5] Patent: WO2015/150476, 2015, A1. Location in patent: Page/Page column 23; 24