1004294-80-7
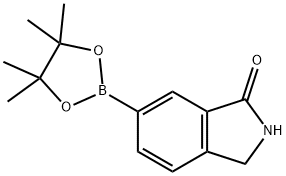 1004294-80-7 结构式
1004294-80-7 结构式
基本信息
1-异吲哚酮-6-硼酸频哪醇酯
1-异吲哚啉酮-6-硼酸频哪醇酯
6-(四甲基-1,3,2-二噁硼戊环-2-基)-2,3-二氢-1H-异吲哚-1-酮
6-(4,4,5,5-四甲基-1,3,2-三氧杂戊硼烷-2-基)-异吲哚啉-1-酮
isoindolin-1-one-6-boronic acid pinacol ester
(3-OXOISOINDOLIN-5-YL)BORONIC ACID PINACOL ESTER
1-Oxo-2,3-dihydro-isoindole-6-boronic acid picol ester
1-Oxo-2,3-dihydro-isoindole-6-boronic acid pinacol ester
6-(4,4,5,5-tetramethyl-1,3-dioxolan-2-yl)isoindolin-1-one
2,3-Dihydro-1H-isoindol-1-one-6-boronic acid pinacol ester
2,3-dihydro-6-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-
(2,3-Dihydro-3-oxo-1H-isoindol-5-yl)boronic acid, pinacol ester
6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)isoindolin-1-one
物理化学性质
制备方法
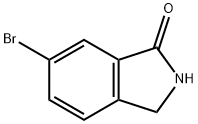
675109-26-9

73183-34-3

1004294-80-7
以6-溴-2,3-二氢-异吲哚-1-酮和联硼酸频那醇酯为原料合成6-(4,4,5,5-四甲基-[1,3,2]二氧杂硼杂环戊烷-2-基)-2,3-二氢-异吲哚-1-酮的一般步骤:向6-溴-2,3-二氢-异吲哚-1-酮(1当量)的无水二恶烷(0.1M)溶液中加入双(频哪醇)二硼(1.1当量)、乙酸钾(3.5当量)和1,1'-双(二苯基膦)二茂铁(dppf,0.05当量)。将反应混合物用氮气脱气20分钟。随后,加入二氯(1,1'-双(二苯基膦)二茂铁)钯(II)(PdCl2(dppf),0.05当量),并将混合物再次脱气5分钟。在氮气保护下,将反应混合物加热至70℃反应2小时,然后升温至120℃继续反应16小时。反应完成后,将混合物在乙酸乙酯(EtOAc)和水之间分配。水相用乙酸乙酯进一步萃取,合并有机相,用硫酸镁(MgSO4)干燥,过滤后真空浓缩。残余物用乙酸乙酯超声处理,悬浮液通过烧结漏斗过滤,收集的灰色固体干燥后可直接用于下一步反应,无需进一步纯化。产物6-(4,4,5,5-四甲基-[1,3,2]二氧杂硼杂环戊烷-2-基)-2,3-二氢-异吲哚-1-酮的收率为82%,纯度为29%,主要杂质为硼酸。LC-MS(ESP)分析显示m/z:519.5 [2M + H]+,保留时间(R/T)为3.38分钟。
参考文献:
[1] Patent: WO2008/23161, 2008, A1. Location in patent: Page/Page column 117
[2] Journal of Medicinal Chemistry, 2013, vol. 56, # 23, p. 9542 - 9555
[3] Patent: WO2017/107089, 2017, A1. Location in patent: Page/Page column 37; 38
[4] Patent: WO2017/68412, 2017, A1. Location in patent: Page/Page column 208; 209
[5] Journal of Medicinal Chemistry, 2018, vol. 61, # 11, p. 4978 - 4992
