1006875-83-7
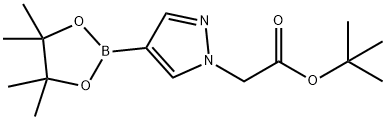 1006875-83-7 结构式
1006875-83-7 结构式
基本信息
2-(4-(4,4,5,5-四甲基-1,3,2-二氧硼戊烷-2-基)-1H-吡唑-1-基)乙酸叔丁酯
Oxirane, 2-[9-(phenylMethoxy)ethyl]-
1-[2-(tert-Butoxy)-2-oxoethyl]-1H-pyrazole-4-boronic Acid Pinacol Ester
tert-butyl 2-[4-(tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazol-1-yl]acetate
tert-butyl 2-[4-(tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl]acetate
tert-butyl [4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl]acetate
tert-Butyl 2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)acetate
1H-Pyrazole-1-acetic acid, 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-, 1,1-dimethylethyl ester
物理化学性质
制备方法
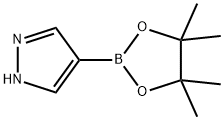
269410-08-4
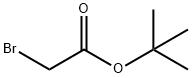
5292-43-3

1006875-83-7
以4-吡唑硼酸频哪醇酯(2.0 g,10.31 mmol)和溴乙酸叔丁酯(2.212 g,11.34 mmol)为原料,在氮气保护下,将混合物与碳酸钾(1.709 g,12.37 mmol)在丙酮(20 mL)中于65℃搅拌反应13小时。反应完成后,将反应混合物倒入冰水(20 mL)中,并用乙酸乙酯(50 mL × 2)萃取。合并有机相,用饱和食盐水洗涤,无水硫酸镁干燥,过滤后减压浓缩。粗产物通过硅胶柱色谱纯化(洗脱剂:石油醚/乙酸乙酯=5/1),得到目标化合物2-(4-(4,4,5,5-四甲基-1,3,2-二氧硼戊烷-2-基)-1H-吡唑-1-基)乙酸叔丁酯(3 g,8.76 mmol,产率85%),为油状物。LC-MS(方法C):m/z 309.2([M+H]+),保留时间:1.946分钟;1H NMR(400 MHz,CDCl3)δ 7.82(s,1H),7.75(s,1H),4.82(s,2H),1.47(s,9H),1.28(s,12H)。
参考文献:
[1] Patent: WO2016/123796, 2016, A1. Location in patent: Page/Page column 49; 50
[2] Patent: US2008/39457, 2008, A1. Location in patent: Page/Page column 36
[3] Patent: US2013/59835, 2013, A1. Location in patent: Paragraph 1146
[4] Patent: US2014/249132, 2014, A1. Location in patent: Paragraph 0424
[5] Patent: US2010/240634, 2010, A1. Location in patent: Page/Page column 57-58
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW02100687583704 | 2-(4-(4,4,5,5-四甲基-1,3,2-二氧硼戊烷-2-基)-1H-吡唑-1-基)乙酸叔丁酯 | 1006875-83-7 | 5G | 2108元 |
| 2025/12/22 | XW02100687583703 | 2-(4-(4,4,5,5-四甲基-1,3,2-二氧硼戊烷-2-基)-1H-吡唑-1-基)乙酸叔丁酯 | 1006875-83-7 | 1G | 613元 |
| 2025/12/22 | XW02100687583702 | 2-(4-(4,4,5,5-四甲基-1,3,2-二氧硼戊烷-2-基)-1H-吡唑-1-基)乙酸叔丁酯 | 1006875-83-7 | 250MG | 234元 |