101376-25-4
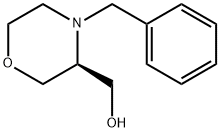 101376-25-4 结构式
101376-25-4 结构式
基本信息
(3S)-4-苄基-3-吗啉甲醇
(S)-4-苄基-3-羟甲基吗啉
(S)-(4-苄基吗啉-3-基)甲醇
(3S)-4-(苯基甲基)-3-吗啉甲醇
(S)-4-BENZYL-3-HYDROXYMETHYLMORPHOLINE
(S)-4-(phenylmethyl)-3-Morpholinemethanol
(3S)-4-(Phenylmethyl)-3-morpholinemethanol
3-Morpholinemethanol, 4-(phenylmethyl)-, (3S)-
(3S)-4-(Phenylmethyl)-3-morpholinemethanol,99%e.e.
(4-Benzyl-3-Morpholinyl)Methanol (4Benzylmorpholin3yl)methanol
物理化学性质
制备方法
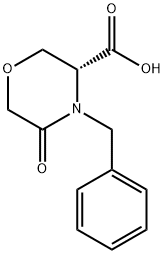
106973-36-8

101376-25-4
以(R)-4-苄基-5-氧代-3-吗啉甲酸为原料合成(S)-(4-苄基吗啉-3-基)甲醇的一般步骤:向搅拌的(R)-4-苄基-5-氧代-3-吗啉甲酸(17.7g,75.3mmol)的四氢呋喃(THF,300mL)溶液中加入三乙胺(NEt3,7.3g,10.0mL,72.0mmol)。将反应混合物冷却至0℃,缓慢加入二甲基硫醚硼烷络合物(BH3·Me2S,10M THF溶液,45.0mL,450.0mmol)。加热回流反应混合物12小时后,冷却至室温,通过缓慢加入甲醇(MeOH)在0℃下淬灭过量的硼烷。将反应混合物真空浓缩,所得白色固体溶于乙酸乙酯(EtOAc,120mL),用氢氧化钠水溶液(20% v/v,2×100mL)洗涤。有机相用2M盐酸水溶液(2×150mL)萃取。合并酸性水相,用固体氢氧化钠碱化至pH 14,再用乙酸乙酯(2×150mL)反萃取。合并有机相,用盐水(150mL)洗涤,无水硫酸镁(MgSO4)干燥,过滤后真空浓缩,得到(S)-(4-苄基吗啉-3-基)甲醇(13.5g,收率87%),为透明油状物,无需进一步纯化。δH(CDCl3)7.29-7.16(5H,m),4.05(1H,d,J=12.8Hz),3.88(1H,dd,J=11.5Hz和J=4.5Hz),3.78(1H,m),3.70-3.53(2H,m),3.51-3.40(2H,m),3.20(1H,d,J=13.2Hz),2.68(1H,dt,J=12.1Hz和J=2.8Hz),2.48(1H,m),2.27(1H,m),2.20-2.15(1H,br.s)。
参考文献:
[1] Patent: WO2008/47109, 2008, A1. Location in patent: Page/Page column 40-41
[2] Patent: WO2009/1089, 2008, A1. Location in patent: Page/Page column 29
[3] Patent: WO2009/71895, 2009, A1. Location in patent: Page/Page column 110
[4] Patent: US2011/3785, 2011, A1. Location in patent: Page/Page column 11
[5] Patent: US2008/255114, 2008, A1. Location in patent: Page/Page column 31
