1020329-80-9
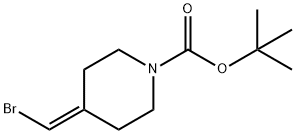 1020329-80-9 结构式
1020329-80-9 结构式
基本信息
1-N-叔丁氧羰基-4-(溴亚甲基)哌啶
1-Boc-4-(Bromomethylene)
1-Boc-4-(BroMoMethylene)piperidine
1-N-Boc-4-(bromomethylene)piperidine
Tert-Butyl4-(bromomethylene)piperidine-1-carboxylate
ert-butyl 4-(bromomethylene)piperidine-1-carboxylate
Tert-butyl 4-(bromomethylene)piperidin-1-carboxylate
tert-butyl 4-(bromomethylidene)piperidine-1-carboxylate
4-Bromomethylene-piperidine-1-carboxylic acid tert-butyl ester
1-Piperidinecarboxylicacid, 4-(bromomethylene)-, 1,1-dimethy...
物理化学性质
制备方法
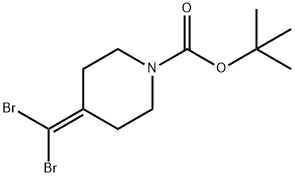
305794-65-4

1020329-80-9
以4-(二溴亚甲基)哌啶-1-甲酸叔丁酯为原料合成1-N-叔丁氧羰基-4-(溴亚甲基)哌啶的一般步骤如下:在氮气保护下,将4-(二溴亚甲基)哌啶-1-甲酸叔丁酯(12g,33.8mmol)溶于甲醇(80ml)中,冷却至0℃。随后,加入四氢呋喃(40ml)和氯化铵(14.46g,270mmol)。反应混合物在0℃下搅拌30分钟后,加入锌粉(8.85g,135mmol)。将反应混合物逐渐升温至室温并继续搅拌4小时。反应完成后,过滤混合物,用甲醇洗涤固体。将滤液减压浓缩,得到白色固体。将该固体溶于乙酸乙酯和水的混合液中,分离有机层和水层。有机层用硫酸镁干燥后,减压浓缩,得到4-(溴亚甲基)哌啶-1-甲酸叔丁酯(9g,收率96%)。产物经1H NMR(400MHz,DMSO-d6)确认:δ6.26(s,1H),3.19-3.43(m,4H),2.15-2.35(m,4H),1.41(s,9H)。质谱(ES+)显示分子离子峰为220和222。
参考文献:
[1] Patent: WO2013/27001, 2013, A1. Location in patent: Page/Page column 61-62
[2] Patent: WO2017/153520, 2017, A1. Location in patent: Page/Page column 55; 56
[3] Patent: US2008/261941, 2008, A1. Location in patent: Page/Page column 37
[4] Patent: WO2009/127943, 2009, A1. Location in patent: Page/Page column 27
[5] Patent: WO2009/127944, 2009, A1. Location in patent: Page/Page column 27
