1029691-06-2
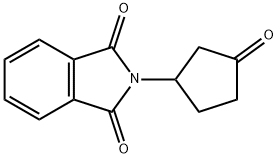 1029691-06-2 结构式
1029691-06-2 结构式
基本信息
2-(3-氧代环戊基)异吲哚啉-1,3-二酮
2-(3-oxocyclopentyl)isoindole-1,3-dione
2-(3-Oxocyclopentyl)isoindoline-1,3-dione
5-(4-hydroxyphenyl)isoxazole-7-carboxylicacid
2-(3-oxocyclopentyl)-1H-Isoindole-1,3(2H)-dione
1H-Isoindole-1,3(2H)-dione, 2-(3-oxocyclopentyl)-
2-(3-oxocyclopentyl)-1H-Isoindole-1,3(2H)-dione1029691-06-2
物理化学性质
制备方法
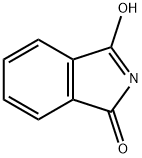
136918-14-4
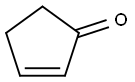
930-30-3

1029691-06-2
以3-羟基-1H-异吲哚-1-酮和2-环戊烯酮为原料合成2-(3-氧代环戊基)异吲哚-1,3-二酮的一般步骤如下:将2-环戊烯酮(100 g,1.2 mol)和3-羟基-1H-异吲哚-1-酮(170 g,1.2 mmol)溶于甲醇(900 mL)中,于室温下搅拌反应18小时。反应过程中使用机械搅拌器进行剧烈搅拌,并缓慢加入2M Na2CO3水溶液(80 mL)。约2小时后,反应体系中逐渐形成浓稠的白色沉淀。继续在室温下搅拌48小时以确保反应完全。反应完成后,通过真空过滤收集白色固体产物,并用甲醇洗涤。将所得固体悬浮于水(300 mL)中,搅拌3小时以去除可溶性杂质。再次收集固体,并于40℃的真空烘箱中干燥过夜,最终得到2-(3-氧代环戊基)异吲哚-1,3-二酮195 g(产率71%),为白色固体。产物经1H NMR(DMSO-d6)表征:δ 7.85-7.77(m,4H),4.90(m,1H),2.67(ddd,1H,J = 18.5, 6.2, 1.3 Hz),2.54(dd,1H,J = 18.5, 9.2 Hz),2.45(m,1H),2.32-2.21(m,3H);质谱(m/z):230(M + 1,弱)。
参考文献:
[1] Patent: US2010/69404, 2010, A1. Location in patent: Page/Page column 12
[2] Patent: US2011/118326, 2011, A1. Location in patent: Page/Page column 8
[3] Patent: WO2009/140448, 2009, A1. Location in patent: Page/Page column 28-20
[4] Patent: WO2017/35357, 2017, A1. Location in patent: Paragraph 0603
[5] Patent: WO2017/35353, 2017, A1. Location in patent: Paragraph 0745