104737-00-0
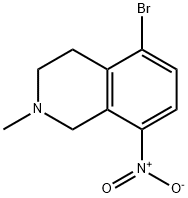 104737-00-0 结构式
104737-00-0 结构式
基本信息
5-bromo-2-methyl-8-nitro-1,2,3,4-tetrahydroisoquinoline
Isoquinoline, 5-bromo-1,2,3,4-tetrahydro-2-methyl-8-nitro-
物理化学性质
制备方法
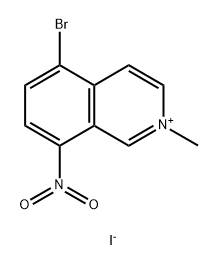
947499-02-7

104737-00-0
在500 mL三颈圆底烧瓶中,将Ni(NO3)2·6H2O(12.6 g,43.33 mmol)溶解于甲醇(200 mL)中。向该溶液中加入5-溴-8-硝基-N-甲基异喹啉碘化物(33.33 g,84.38 mmol)。随后,分批加入NaCNBH3(10.6 g,168.68 mmol)。将反应混合物在室温下搅拌5小时,反应进程通过薄层色谱(TLC)监测(展开剂比例为EtOAc:PE = 1:5)。反应完成后,使用旋转蒸发器在减压下浓缩反应液。将残余物溶解于800 mL水中,并用5% NaOH溶液调节pH至8-10。过滤后,用800 mL EtOAc萃取水相两次,合并有机层并用Na2SO4干燥。最后,通过柱色谱法(洗脱剂比例为EtOAc:PE = 1:5)纯化残余物,得到19.3 g(产率83%)的5-溴-2-甲基-8-硝基-1,2,3,4-四氢异喹啉,为黄色固体。
参考文献:
[1] Patent: US2008/318941, 2008, A1. Location in patent: Page/Page column 23
[2] Patent: WO2009/23844, 2009, A2. Location in patent: Page/Page column 105-106
[3] Patent: US2008/200471, 2008, A1. Location in patent: Page/Page column 45; 46
[4] Patent: US2010/16297, 2010, A1. Location in patent: Page/Page column 25
[5] Patent: WO2010/21797, 2010, A1. Location in patent: Page/Page column 69