1049004-32-1
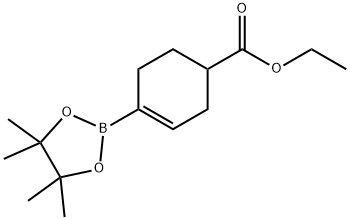 1049004-32-1 结构式
1049004-32-1 结构式
基本信息
4-(乙氧羰基)环己烯-1-硼酸频哪醇酯
1-乙氧基羰基环己-3-烯-4-硼酸频哪醇酯
ETHYL4-(4,41-乙氧基羰基环己-3-烯-4-硼酸频哪醇酯
4-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)-3-环己烯E-1-羧酸乙酯
4-(4,4,5,5-四甲基-[1,3,2]二氧杂硼杂环戊烷-2-基)环己-3-烯-1-羧基LIC ACID 乙基 酯
1-Ethoxycarbonylcyclohex-3-ene-4-boronic acid pinacol ester
4-(Ethoxycarbonyl)cyclohexene-1-boronic acid, pinacol ester
4-(ETHOXY(HYDROXY)BORYL)CYCLOHEX-3-ENECARBOXYLATE PINACOL ESTER
4-(Ethoxycarbonyl)cyclohexene-1-boronic acid, pinacol ester 97%
Ethyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3-cycl...
ethyl 4-(hydroxy((3-hydroxy-2,3-dimethylbutan-2-yl)oxy)boryl)cyclohex-
ethyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)cyclohex-3-enecarboxylate
ethyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)cyclohex-3-ene-1-carboxylate
Ethyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3-cyclohexene-1-carboxylate
物理化学性质
制备方法
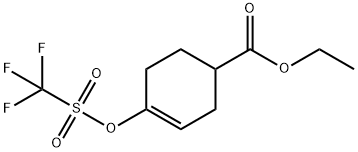
122948-57-6

73183-34-3

1049004-32-1
以4-((三氟甲烷磺酰基)氧基)-3-环己烯-1-羧酸乙酯(303 mg,1 mmol)和联硼酸频那醇酯(370 mg,1.5 mmol)为原料,加入KOAc(400 mg,4 mmol)和Pd(dppf)Cl2(10 mg,0.05 mmol)于二恶烷(5 mL)中。将反应混合物在110℃下加热16小时。反应完成后,冷却至室温,将混合物在EtOAc(10 mL)和饱和NaHCO3溶液(10 mL)之间分配。水相用EtOAc(10 mL × 2)萃取。合并的有机相用盐水洗涤,经无水MgSO4干燥,过滤,浓缩。通过快速色谱法(25% EtOAc的石油醚溶液)纯化,得到4-硼酸频哪醇酯-3-环己烯甲酸乙酯(250 mg,收率89%)。LRMS m/z (M + H)+: 281(实测值),281(计算值)。
参考文献:
[1] Patent: WO2014/81619, 2014, A1. Location in patent: Page/Page column 128
[2] Patent: US2011/251183, 2011, A1. Location in patent: Page/Page column 18
[3] Patent: US2011/263578, 2011, A1. Location in patent: Page/Page column 34
[4] Patent: WO2011/128265, 2011, A1. Location in patent: Page/Page column 34
[5] Patent: US2011/275801, 2011, A1. Location in patent: Page/Page column 49
