105258-93-3
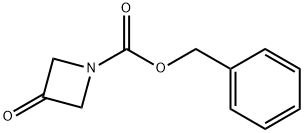 105258-93-3 结构式
105258-93-3 结构式
基本信息
N-CBZ-3-氮杂环丁酮
苄氧羰基氮杂环丁烷-3-酮
3-氧代吖丁啶-1-羧酸苄酯
N-CBZ-氮杂环丁烷-3-酮
1-苄氧羰基氮杂环丁烷-3-酮
1-苄氧羰基-3-酮氮杂环丁烷
1-CBZ-氮杂环丁烷-3-酮
1-苄氧羰基-3-氧代氮杂环丁烷
3-氧代氮杂环丁烷-1-甲酸苄酯
N-CBZ-3-OXOAZETIDINE
Azetidin-3-one, N-CBZ protected
1-(Benzyloxycarbonyl)azetidin-3-one
3-Oxo-azetidine-1-carboxylic acid benzyl ester
3-oxo-1-azetidinecarboxylic acid (phenylmethyl) ester
1-Azetidinecarboxylic acid, 3-oxo-, phenylMethyl ester
Benzyl 3-oxoazetidine-1-carboxylate, 1-[(Benzyloxy)carbonyl]-3-oxoazetidine
物理化学性质
制备方法
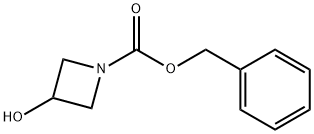
128117-22-6

105258-93-3
以1-苄氧羰基-3-羟基氮杂环丁烷为原料合成1-苄氧羰基氮杂环丁烷-3-酮的一般步骤:在室温条件下,将三苯基甲基-3-羟基氮杂环丁烷-1-羧酸酯(3.5g,0.0168mol)溶解于二氯甲烷(100mL)中,随后加入Dess-Martin periodinane(10.7g,0.025mol)。反应混合物在室温下搅拌5小时。反应完成后,用1:1比例的饱和碳酸氢钠水溶液和1M硫代硫酸钠溶液(200mL)淬灭反应。混合物用二氯甲烷萃取,分离有机层。有机层用无水硫酸镁干燥,随后在减压条件下浓缩,得到3-氧代氮杂环丁烷-1-羧酸苯基甲酯(3.43g,收率99%),产物为透明无色油状物,无需进一步纯化。产物经1H NMR(400MHz,CDCl3)确认:δ 7.39-7.31(m,5H),5.17(s,2H),4.77(s,4H)。质谱(EI)分析结果与C11H11NO3的分子量相符:205(M+)。
参考文献:
[1] Patent: WO2007/44515, 2007, A1. Location in patent: Page/Page column 165
[2] Patent: WO2008/76415, 2008, A1. Location in patent: Page/Page column 329
[3] Patent: WO2008/124085, 2008, A2. Location in patent: Page/Page column 179
[4] Patent: WO2014/32, 2014, A1. Location in patent: Page/Page column 33; 34
[5] Patent: WO2013/8002, 2013, A1. Location in patent: Page/Page column 49
