10538-65-5
中文名称
(3ALPHA,5BETA,12ALPHA)-3,12-二羟基-7-酮基胆烷-24-酸甲酯
英文名称
(3alpha,5beta,12alpha)-3,12-Dihydroxy-7-oxocholan-24-oic acid methyl ester
CAS
10538-65-5
分子式
C25H40O5
分子量
420.58
MOL 文件
10538-65-5.mol
更新日期
2026/02/05 13:33:19
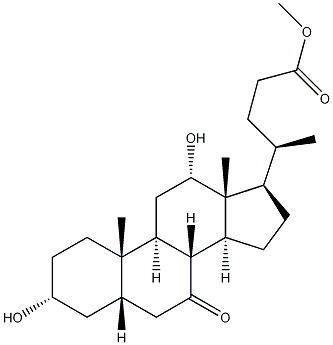 10538-65-5 结构式
10538-65-5 结构式
基本信息
中文别名
3Α,12Α-二羟基,7酮基-5Β-胆烷酸甲酯甲基-7-氧代-3A,12A-二羟基-5B-胆酸酯
(3Α,5Β,12Α)-3,12-二羟基-7-酮基胆烷-24-酸甲酯
(3ALPHA,5BETA,12ALPHA)-3,12-二羟基-7-酮基胆烷-24-酸甲酯
英文别名
METHYL 7-KETOCHOLATEMethyl-7-keto-3a,12a-dihydroxy-5b-cholanoate
3α,12α-diol-7-oxo-5β-24-cholanoic acid methyl ester, methyl
3α,12α-diol-7-oxo-5β-24-cholanoic acid Methyl ester, Methyl
(3a,5b,12a)-3,12-Dihydroxy-7-oxocholan-24-oic acid methyl ester
(3α,5β,12α)-3,12-Dihydroxy-7-oxocholan-24-oic acid Methyl ester
(3α,5β,12α)-3,12-Dihydroxy-7-oxocholan-24-oic acid methyl ester
Cholan-24-oic acid, 3,12-dihydroxy-7-oxo-, methyl ester, (3α,5β,12α)-
(3alpha,5beta,12alpha)-3,12-Dihydroxy-7-oxocholan-24-oic acid methyl ester
methyl (R)-4-((3R,5S,8R,9S,10S,12S,13R,14S,17R)-3,12-dihydroxy-10,13-dimethyl-7-oxohexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate
所属类别
化学试剂:甲酯类化合物制备方法
方法1
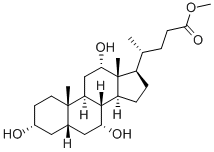
1448-36-8
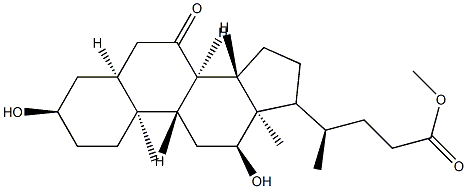
15073-97-9
以(R)-甲基 4-((3R,5S,7R,8R,9S,10S,12S,13R,14S,17R)-3,7,12-三羟基-10,13-二甲基十六氢-1H-环戊[a]菲蒽17-基)戊酸酯为起始原料,首先通过保护胆酸羟基得到甲基酯(2)。随后,使用N-溴代琥珀酰亚胺(NBS)将甲基酯(2)氧化为相应的酮(3)。接着,将酮(3)作为二乙酸酯(4)进行保护,并通过与分子溴反应转化为关键中间体溴化物(5)。之后,将溴化物(5)水解生成羟基酮(6)。最后,通过还原乙酸酯和甲酯部分,并进行全面脱保护,得到目标产物(R)-4-((3R,5S,8R,9S,10S,12S,13R,14S,17R)-3,12-二羟基-10,13-二甲基-7-氧代十六氢-1H-环戊并[a]菲-17-基)戊酸甲酯(7)。
参考文献:
[1] Advanced Synthesis and Catalysis, 2012, vol. 354, # 14-15, p. 2821 - 2828
[2] Journal of the Chemical Society, Chemical Communications, 1993, # 19, p. 1469 - 1471
[3] Patent: WO2011/22838, 2011, A1. Location in patent: Page/Page column 37
[4] Journal of Biological Chemistry, 1943, vol. 147, p. 131,133
[5] Steroids, 1993, vol. 58, # 2, p. 52 - 58