105544-30-7
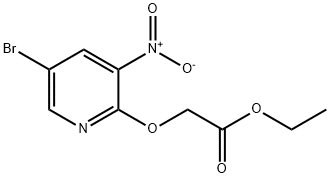 105544-30-7 结构式
105544-30-7 结构式
基本信息
2-[(5-溴-3-硝基-2-吡啶基)氧基]乙酸乙酯
ethyl 2-(5-bromo-3-nitropyridin-2-yloxy)acetate
5-Bromo-3-nitro-pyridin-2-yloxy)-acetic acid ethyl ester
Acetic acid, 2-[(5-bromo-3-nitro-2-pyridinyl)oxy]-, ethyl ester
物理化学性质
制备方法
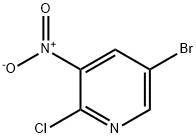
67443-38-3
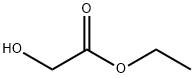
623-50-7

105544-30-7
方法I:在干燥条件下,将5-溴-2-氯-3-硝基吡啶(100 mg,0.42 mmol)和乙醇酸乙酯(0.044 mL,0.46 mmol)溶解于无水THF(5 mL)中,并将混合物冷却至0℃。在氮气保护下,缓慢加入氢化钠(60%分散于矿物油中,0.015 mL,0.46 mmol),随后将反应混合物逐渐升温至室温并搅拌1小时。反应完成后,将混合物小心倒入冰水中,并用乙酸乙酯(2×10 mL)萃取。合并有机层,用饱和食盐水(10 mL)洗涤,无水硫酸钠干燥,过滤后减压浓缩。粗产物通过快速柱色谱法(硅胶100-200目)纯化,得到2-(5-溴-3-硝基吡啶-2-基氧基)乙酸乙酯(105 mg,收率82%)。产物表征数据如下:1H NMR (300 MHz, DMSO-d6) δ = 1.27 (s, 3H), 4.25 (q, 2H), 5.12 (s, 2H), 8.40 (d, J = 2.07 Hz, 1H), 8.47 (d, J = 2.07 Hz, 1H); ESMS: m/z 306.8 [M + 1]。
参考文献:
[1] Synthetic Communications, 2013, vol. 43, # 24, p. 3315 - 3321
[2] Patent: US2007/191603, 2007, A1. Location in patent: Page/Page column 37
[3] Patent: WO2011/77098, 2011, A1. Location in patent: Page/Page column 157-158
[4] Patent: WO2007/77961, 2007, A2. Location in patent: Page/Page column 358