106429-57-6
中文名称
2-氧代-2,3-二氢-1H-1,3-苯并咪唑-5-羧酸甲酯
英文名称
5-CARBOXYBENZIMIDAZOLONE METHYL ESTER
CAS
106429-57-6
分子式
C9H8N2O3
分子量
192.17
MOL 文件
106429-57-6.mol
更新日期
2025/12/01 11:58:04
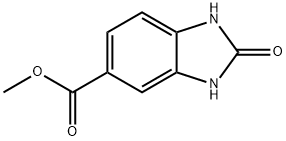 106429-57-6 结构式
106429-57-6 结构式
基本信息
中文别名
5-羧基苯并咪唑酮甲酯2-氧代-2,3-二氢苯并咪唑-5-甲酸甲酯
2-氧代-2,3-二氢-1H-苯并[D]咪唑-5-羧酸甲酯
2-氧代-2,3-二氢-1H-1,3-苯并咪唑-5-羧酸甲
1H-苯并咪唑-5-甲酸, 2,3-二氢-2-氧代-, 甲酯
2-氧代-2,3-二氢-1H-1,3-苯并咪唑-5-羧酸甲酯
英文别名
5-CARBOXYBENZIMIDAZOLONE METHYL ESTERMethyl 2-Oxo-2,3-dihydrobenzimidazole-5-carboxylate
Methyl 2-oxo-1,3-dihydro-1,3-benzodiazole-5-carboxylate
methyl 2-oxo-2,3-dihydro-1H-1,3-benzodiazole-5-carboxylate
methyl 2-oxo-2,3-dihydro-1H-1,3-benzimidazole-5-carboxylate
Methyl 2-oxo-2,3-dihydro-1H-benzo[d]iMidazole-5-carboxylate
2-Oxo-2,3-dihydro-1H-benzoimidazole-5-carboxylic acid methyl ester
1H-Benzimidazole-5-carboxylic acid, 2,3-dihydro-2-oxo-, methyl ester
1H-Benzimidazole-5-carboxylicacid,2,3-dihydro-2-oxo-,methylester(9CI)
所属类别
医药中间体:杂环化合物物理化学性质
熔点312-313°
沸点221.2±19.0 °C(Predicted)
密度1.325±0.06 g/cm3(Predicted)
储存条件2-8°C
酸度系数(pKa)11.54±0.30(Predicted)
外观Light brown to gray Solid
制备方法
方法1
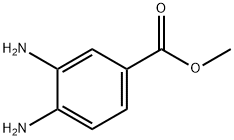
36692-49-6
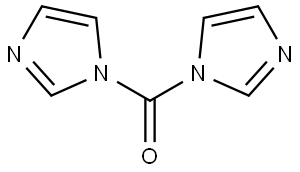
530-62-1

106429-57-6
在0℃下,将3,4-二氨基苯甲酸甲酯(5.00g,30.1mmol)溶于THF(40mL)中,随后加入N,N'-羰基二咪唑(7.32g,45.1mmol)。反应混合物在0℃下搅拌16小时,并逐渐升温至23℃。反应完成后,在0℃下缓慢加入1M HCl水溶液(50mL),接着加入水(70mL),继续搅拌1小时。通过过滤收集所得沉淀,并在减压下干燥18小时,得到2-氧代-2,3-二氢-1H-苯并[d]咪唑-5-羧酸甲酯(5.45g,收率94%),产物无需进一步纯化即可用于后续反应。质谱(ESI/CI):计算值C9H8N2O3,192.1;实测值m/z 193.1 [M+H]+。1H NMR(400MHz,CDCl3)δ:11.01(s,1H),10.84(s,1H),7.63(dd,J=8.2,1.6Hz,1H),7.47(s,1H),7.02(d,J=8.2Hz,1H),3.82(s,3H)。
参考文献:
[1] Patent: WO2009/134750, 2009, A1. Location in patent: Page/Page column 139
[2] Journal of Medicinal Chemistry, 2006, vol. 49, # 12, p. 3719 - 3742
[3] Patent: US2010/249124, 2010, A1. Location in patent: Page/Page column 62
[4] Cell Chemical Biology, 2018, vol. 25, # 6, p. 677 - 12,690
