1089687-05-7
中文名称
2,6-二溴-4,4-双(2-乙基己基)-4H-硅杂环戊二烯并[3,2-B:4,5-B']二噻吩
英文名称
2,6-Dibromo-4,4-bis(2-ethylhexyl)-4H-silolo[3,2-b:4,5-b']dithiophene
CAS
1089687-05-7
分子式
C24H36Br2S2Si
分子量
576.57
MOL 文件
1089687-05-7.mol
更新日期
2026/01/28 08:44:00
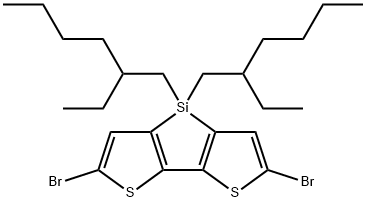 1089687-05-7 结构式
1089687-05-7 结构式
基本信息
中文别名
M80212,6-二溴-4,4-二(2-乙基己基)-二噻吩并噻咯
[2,6-二溴-4,4-双(2-乙基己基)-4H-硅杂环戊二烯并[3,2-B
2,6-二溴-4,4-双(2-乙基己基)-4H-硅杂环戊二烯并[3,2-b:4,5-b’]二噻吩
2,6-二溴-4,4-双(2-乙基己基)-4H-硅杂环戊二烯并[3,2-B:4,5-B']二噻吩
4,4'-双(2-乙基-己基)-5,5'-二溴-二噻吩并[3,2-B:2',3'-D]硅杂环戊二烯
5,5'-二溴-4,4'-二(2-乙基己基)-4H-硅杂环戊二烯并[3,2-B:4,5-B']二噻吩
2,6-二溴-4,4-双(2-乙基己基)-4H-硅杂环戊二烯并[3,2-B:4,5-B']二噻吩 1G
2,6-二溴-4,4-双(2-乙基己基)-4H-硅杂环戊二烯并(3,2-B:4,5-B')二噻吩 >97%
2,6-二溴-4,4-双(2-乙基己基)-4H-硅杂环戊二烯并[3,2-B:4,5-B']二噻吩2,6-DIBROMO-4,4-BIS(2-ETHYLHEXYL)-4H-THIENO[2',3':4,5]SILOLO[3,2- B]THIOPHENECAS: 1089687-05-7
英文别名
DTS26-2Br2,6-Dibromo-4,4-bis(2-ethylhexyl)-4H-silolo[3,2-b
4,4'-Bis(2-ethyl-hexyl)-5,5'-dibroMo-dithieno[3,2-b:2',3'-d
2,6‐dibroMo‐(4,4‐di‐2‐ ethylhexyldithieno[ 3,2‐b:2',3'‐ d]silole
4,4'-Bis(2-ethyl-hexyl)-5,5'-dibroMo-dithieno[3,2-b:2',3'-d]silole
2,6-Dibromo-4,4-bis(2-ethylhexyl)-4H-silolo[3,2-b:4,5-b']dithiophene
4H-Silolo[3,2-b:4,5-b']dithiophene, 2,6-dibroMo-4,4-bis(2-ethylhexyl)-
2,6-Dibromo-4,4-bis(2-ethylhexyl)-4H-silolo[3,2-b:4,5-b']dithiophene
2,6-Dibromo-4,4-bis(2-ethylhexyl)-4H-thieno[2',3':4,5]silolo[3,2- b]thiophene
物理化学性质
沸点551.3±50.0 °C(Predicted)
密度1.32
储存条件Keep in dark place,Inert atmosphere,2-8°C
InChIInChI=1S/C24H36Br2S2Si/c1-5-9-11-17(7-3)15-29(16-18(8-4)12-10-6-2)19-13-21(25)27-23(19)24-20(29)14-22(26)28-24/h13-14,17-18H,5-12,15-16H2,1-4H3
InChIKeyCMMVJTQAECTXDZ-UHFFFAOYSA-N
SMILESC12C3SC(Br)=CC=3[Si](CC(CC)CCCC)(CC(CC)CCCC)C=1C=C(Br)S2
制备方法
方法1
![4H-Silolo[3,2-b:4,5-b']dithiophene, 4,4-bis(2-ethylhexyl)-2,6-bis(trimethylsilyl)-](/CAS/20200611/GIF/1089687-04-6.gif)
1089687-04-6
![2,6-二溴-4,4-双(2-乙基己基)-4H-硅杂环戊二烯并[3,2-B:4,5-B']二噻吩](/CAS/20200611/GIF/1089687-05-7.gif)
1089687-05-7
以化合物(CAS:1089687-04-6)为起始原料,合成2,6-二溴-4,4-双(2-乙基己基)-4H-硅杂环戊二烯并[3,2-B:4,5-B']二噻吩的一般步骤如下:在10℃条件下,将N-溴代琥珀酰亚胺(NBS)(11.65g,65.42mmol,2.06当量)缓慢加入至溶解于350ml无水四氢呋喃中的化合物(17.885g,31.76mmol)溶液中。反应混合物在室温下搅拌过夜。反应完成后,通过硅胶柱色谱法(洗脱剂:正己烷)对粗产物进行纯化,得到黄色油状的目标化合物17a(16.47g,收率90%)。
参考文献:
[1] Journal of the American Chemical Society, 2008, vol. 130, # 48, p. 16144 - 16145
[2] Chemical Communications, 2009, # 37, p. 5570 - 5572
[3] Patent: KR2017/50088, 2017, A. Location in patent: Paragraph 0237; 0238
[4] Patent: EP2530085, 2012, A1. Location in patent: Page/Page column 11-12
[5] Organic Electronics: physics, materials, applications, 2016, vol. 39, p. 361 - 370