1092539-44-0
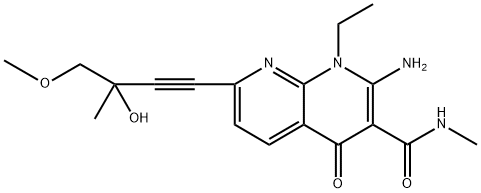 1092539-44-0 结构式
1092539-44-0 结构式
基本信息
SAR131675外消旋体
VEGFR3抑制剂(SAR131675)
(-)-2-氨基-1-乙基-1,4-二氢-7-(3-羟基-4-甲氧基-3-甲基-1-丁炔-1-基)-N-甲基-4-氧代-1,8-萘啶-3-甲酰胺
(Rac)-SAR131675
SAR131675 Racemate
SAR131675/SAR-131675
(R)-2-Amino-1-ethyl-7-(3-hydroxy-4-methoxy-3-methylbut-1-yn-1-yl)-N-methyl-4-oxo-1,4-dihydro-1
2-Amino-1-ethyl-7-(3-hydroxy-4-methoxy-3-methyl-1-butyn-1-yl)-n-methyl-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxamide
(-)-2-Amino-1-ethyl-1,4-dihydro-7-(3-hydroxy-4-methoxy-3-methyl-1-butyn-1-yl)-N-methyl-4-oxo-1,8-naphthyridine-3-carboxamide
1,8-Naphthyridine-3-carboxamide, 2-amino-1-ethyl-1,4-dihydro-7-(3-hydroxy-4-methoxy-3-methyl-1-butyn-1-yl)-N-methyl-4-oxo-, (-)-
物理化学性质
制备方法
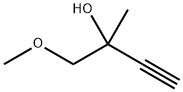
42841-64-5
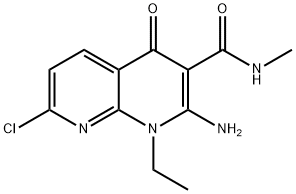
1092523-24-4

1092538-80-1
在20 mL甲醇中悬浮3 g(10.69 mmol)2-氨基-7-氯-1-乙基-N-甲基-4-氧代-1,4-二氢[1,8]二氮杂萘-3-甲酰胺,置于80 mL微波管中。向悬浮液中加入DMF/Et3N混合液(V/V; 1/1),随后用氩气鼓泡10分钟。依次加入1.83 g(±)-1-甲氧基-2-甲基-3-丁炔-2-醇(16.03 mmol)、0.081 g CuI(0.43 mmol)和0.375 g双(三苯基膦)二氯化钯(II)(0.53 mmol)。将密封管置于微波反应器(CEM Discover型号)中,在90℃、100 W功率下加热60分钟。反应完成后,冷却混合物并蒸发至干。将残余物溶解于乙酸乙酯/THF混合溶剂中,用0.1 N HCl水溶液洗涤。有机相经无水硫酸钠干燥,过滤后真空浓缩。通过硅胶柱色谱纯化残余物(干法上样,洗脱梯度:环己烷/乙酸乙酯,30:70至20:80),得到2.49 g浅黄色固体产物。产物可经乙醇重结晶得白色晶体,熔点为211℃。质谱(MH+): 358。产率: 65%。1H NMR(DMSO-d6, 400 MHz, δ ppm): δ 11.75(s, <1H, 宽峰); 11.00(q, 1H, 宽峰); 8.45(d, 1H); 8.00(s, 1H, 宽峰); 7.4(d, 1H); 5.8(s, 1H); 4.4(q, 2H); 3.5-3.3(m + s, 5H); 2.8(d, 3H); 1.45(s, 3H); 1.2(t, 3H)。
参考文献:
[1] Patent: US2010/144757, 2010, A1. Location in patent: Page/Page column 8
