111851-98-0
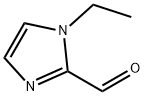 111851-98-0 结构式
111851-98-0 结构式
基本信息
N-乙基-2-咪唑甲醛
1-乙基咪唑-2-甲醛
1-乙基-2-咪唑甲醛
1-乙基咪唑-2-甲醛,95%
1-乙基-1H-咪唑-2-甲醛
1-ethylimidazole-2-carbaldehyde
1-ethyl-2-imidazolecarboxaldehyde
N-Ethyl-2-imidazolecarboxaldehyde
1-Ethylimidazole-2-carboxaldehyde
1-Ethylimidazole-2-carboxaldehyde,95%
1-Ethyl-1H-imidazole-2-carboxaldehyde
1H-IMidazole-2-carboxaldehyde, 1-ethyl-
1H-Imidazole-2-carboxaldehyde,1-ethyl-(9CI)
1-ethyl-1H-imidazole-2-carbaldehyde(SALTDATA: FREE)
物理化学性质
制备方法

75-03-6
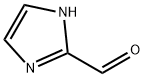
10111-08-7

111851-98-0
向1H-咪唑-2-甲醛(480 mg,5 mmol)和无水碳酸钾(936 mg,6 mmol)在N,N-二甲基甲酰胺(7 mL)中的悬浮液中加入碘乙烷(829 mg,6 mmol)。将反应混合物在50℃下搅拌加热5小时。反应完成后,减压蒸馏除去溶剂。将残余物用蒸馏水(30 mL)和乙酸乙酯(30 mL)进行液液分配。水相用乙酸乙酯(20 mL×3)进一步萃取。合并所有有机相,用饱和氯化钠溶液洗涤,无水硫酸钠干燥,减压浓缩,得到1-乙基-1H-咪唑-2-甲醛,为浅黄色油状物(520 mg,收率84%)。产物经1H NMR(400 MHz,CDCl3)表征:δ 1.42-1.46(t,J = 7.2 Hz,3H),4.42-4.47(q,2H),7.19(s,1H),7.29(s,1H),9.82(s,1H)。LC-MS(ESI)m/z:125(M + 1)+。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2005, vol. 13, # 2, p. 363 - 386
[2] Patent: US2010/35883, 2010, A1. Location in patent: Page/Page column 70-71
[3] Patent: EP1422228, 2004, A1. Location in patent: Page 223
[4] Patent: EP1550657, 2005, A1. Location in patent: Page/Page column 84
[5] Chemico-Biological Interactions, 2016, vol. 259, p. 85 - 92
