112257-12-2
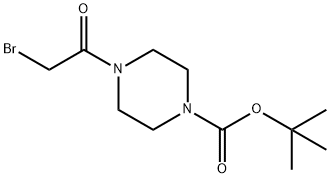 112257-12-2 结构式
112257-12-2 结构式
基本信息
1-BOC-4-(2-溴乙酰基)哌嗪
4-(2-溴乙酰基)哌嗪-1-羧酸叔丁酯
1-Boc-4-(2-bromoacetyl)piperazine
1-(Bromoacetyl)-4-(tert-butoxycarbonyl)piperazine
tert-Butyl 4-(bromoacetyl)-1-piperazinecarboxylate
tert-Butyl 4-(bromoacetyl)piperazine-1-carboxylate
tert-butyl 4-(2-broMoacetyl)piperazine-1-carboxylate
4-(Bromoacetyl)-1-piperazinecarboxylic acid tert-butyl ester
4-(2-Bromoacetyl)-1-Piperazinecarboxylic acid 1,1-dimethylethyl ester
1-Piperazinecarboxylic acid, 4-(bromoacetyl)-, 1,1-dimethylethyl ester
1-Piperazinecarboxylic acid, 4-(2-bromoacetyl)-, 1,1-dimethylethyl ester
物理化学性质
制备方法
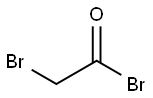
598-21-0
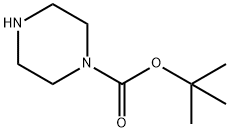
57260-71-6

112257-12-2
在惰性气氛下,将反应体系冷却至-78℃,缓慢向溶解于二氯甲烷(150 mL)中的N-BOC-哌嗪(53.7 mmol,1当量)和三乙胺(59.1 mmol,1.1当量)溶液中滴加溴乙酰溴(53.7 mmol,1当量)。反应混合物在-78℃下持续搅拌3小时,随后用二氯甲烷(75 mL)稀释,并用去离子水洗涤。分离有机层,用无水硫酸镁干燥,减压浓缩除去溶剂。粗产物用乙醚重结晶,过滤后真空干燥,得到4-(2-溴乙酰基)哌嗪-1-羧酸叔丁酯(6q)。产物为白色固体,收率78%,熔点23-242℃。分子量:307.18 g/mol。红外光谱(KBr, cm?1):2965(m),1689(s),1632(s),1417(s),1246(s),1167(s),1023(m)。核磁共振氢谱(250 MHz, CDCl?)δ:3.87(s, 2H),3.61-3.57(m, 2H),3.55-3.47(m, 4H),3.46-3.41(m, 2H),1.46(s, 9H)。核磁共振碳谱(63 MHz, CDCl?)δ:165.5(Cq),154.5(Cq),80.5(Cq),46.6(2CH?),40.9(2CH?),28.4(3CH?),25.7(CH?)。
参考文献:
[1] Journal of the American Chemical Society, 2008, vol. 130, # 2, p. 556 - 565
[2] Beilstein Journal of Organic Chemistry, 2013, vol. 9, p. 1407 - 1413
[3] Patent: US2016/168156, 2016, A1. Location in patent: Paragraph 0510; 0511
[4] Patent: WO2014/57266, 2014, A1. Location in patent: Page/Page column 61
[5] Tetrahedron, 1998, vol. 54, # 16, p. 3999 - 4012