1139453-98-7
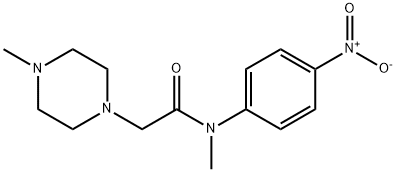 1139453-98-7 结构式
1139453-98-7 结构式
基本信息
英丹尼布杂质12
尼达尼布标准品003
N-(4-硝基苯基)-N,4-二甲基-1-哌嗪乙酰胺
N,4-二甲基-N-(4-硝基苯基)-1-哌嗪乙酰胺
N,4-二甲基-N-(4-硝基苯基)-1-哌嗪乙酰胺 10G
N-甲基-2-(4-甲基哌嗪-1-基)-N-(4-硝基苯基)乙酰胺
N-甲基-2-(4-甲基-1-哌嗪基)-N-(4-硝基苯基)乙酰胺
尼达尼布杂质12:N-甲基-2-(4-甲基哌嗪-1-基)-N-(4-硝基苯基)-乙酰胺
N-METHYL-2-(4-METHYLPIPERAZIN-1-YL)-N-(4-NITROPHENYL)ACET...
Intedanib Impurity 12
Nintedanib Intermediate 1
N,4-Dimethyl-N-(4-nitrophenyl)-1-piperazineacetamide
1-Piperazineacetamide, N,4-dimethyl-N-(4-nitrophenyl)-
N-methyl-2-(4-methylpiperazin-1-yl)-N-(4-nitrophenyl)acet...
N-methyl-2-(4-methylpiperazin-1-yl)-N-(4-nitrophenyl)acetamide
N-Methyl-2-(4-methyl-1-piperazinyl)-N-(4-nitrophenyl)acetamide
N-methyl-2-(4-methylpiperazin-1-yl)-N-(4-nitrophenyl)acetami...
N,4-Dimethyl-N-(4-Nitrophenyl)-1-Piperazineacetamide(Under Development)
物理化学性质
制备方法
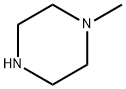
109-01-3
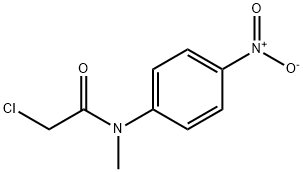
2653-16-9

1139453-98-7
以N-甲基哌嗪和2-氯-N-甲基-N-(4-硝基苯基)乙酰胺为原料合成N-甲基-2-(4-甲基哌嗪-1-基)-N-(4-硝基苯基)乙酰胺的一般步骤:将1-甲基哌嗪(7.2 mL,65 mmol)和碳酸钾(13.8 g,100 mmol)溶于丙酮(200 mL)中,随后缓慢加入2-氯-N-甲基-N-(4-硝基苯基)乙酰胺(11.4 g,50 mmol)。将反应混合物在室温下搅拌12小时。反应完成后,过滤除去沉淀,滤液经旋转蒸发去除溶剂。将残余物溶解于乙酸乙酯(50 mL × 3)中,并用20 mL水进行萃取。有机相用无水硫酸钠干燥后,减压浓缩除去溶剂,得到15 g目标化合物。LC-MS分析显示:m/z = 293([M+H]+)。
参考文献:
[1] Patent: WO2009/71524, 2009, A2. Location in patent: Page/Page column 21-22; 13
[2] Patent: WO2009/71524, 2009, A2. Location in patent: Page/Page column 21-22; 13
[3] Patent: WO2009/71523, 2009, A1. Location in patent: Page/Page column 4; 23-24
[4] Patent: WO2009/71523, 2009, A1. Location in patent: Page/Page column 4; 24
[5] Journal of Labelled Compounds and Radiopharmaceuticals, 2015, vol. 58, # 7, p. 308 - 312
