114435-86-8
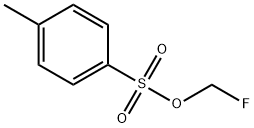 114435-86-8 结构式
114435-86-8 结构式
基本信息
4-甲基苯磺酸氟甲酯
4-氟甲基苯磺酸甲酯
氟甲基-4-甲基苯磺酸酯
4-甲基苯磺酸(氟甲基)酯
luoroMethyl 4-Methylbenzenesulfonate
fluoroMethyl 4-Methylbenzenesulfonate
Fluoromethyl 4-methylbenzenesulfonate 97+%
Methanol, fluoro-, 4-methylbenzenesulfonate
Methanol, 1-fluoro-, 1-(4-methylbenzenesulfonate)
物理化学性质
制备方法
![1-甲基-4-[(4-甲基苯基)磺酰氧基甲氧基磺酰基]苯](/CAS/GIF/24124-59-2.gif)
24124-59-2

114435-86-8
以亚甲基双(4-甲基苯磺酸酯)为原料合成氟甲基-4-甲基苯磺酸酯的一般步骤:将亚甲基双(4-甲基苯磺酸酯)(100 mg,0.28 mmol)和氟化铯(213 mg,1.4 mmol)溶解于叔戊醇(5 mL)中,置于微波反应器中,于90℃下反应15分钟。反应完成后,冷却至室温,减压蒸馏除去叔戊醇。向残余物中加入冰冷的乙醚,过滤所得悬浮液,并用大量冰冷的乙醚洗涤固体。滤液经减压浓缩,得到4-甲基苯磺酸氟甲酯,为无色油状物(49 mg,收率88%)。产物结构经1H NMR(500 MHz,CDCl3)δ:2.43(s,3H,ArCH3),5.72(d,2H,CH2,J = 51.0 Hz),7.35(d,2H,ArH,J = 8.0 Hz),7.81(d,2H,ArH,J = 8.0 Hz);13C NMR(125 MHz,CDCl3)δ:21.67,98.16(d,J = 221 Hz),127.87,129.96,133.77,145.63;19F NMR(471 MHz,CDCl3)δ:-153.24(t,J = 50.7 Hz)确认。
参考文献:
[1] Tetrahedron Letters, 2018, vol. 59, # 17, p. 1635 - 1637
[2] Bioorganic and Medicinal Chemistry Letters, 2015, vol. 25, # 18, p. 3956 - 3960
[3] Journal of Labelled Compounds and Radiopharmaceuticals, 2003, vol. 46, # 6, p. 555 - 566
[4] ChemMedChem, 2016, vol. 11, # 1, p. 108 - 118
[5] Patent: WO2012/40133, 2012, A2. Location in patent: Page/Page column 36-37

