1173081-96-3
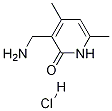 1173081-96-3 结构式
1173081-96-3 结构式
基本信息
3-(氨甲基)-4,6-二甲基吡啶-2(1H)-酮盐酸盐
3-(氨基甲基)-4,6-二甲基吡啶-2(1H)-酮盐酸盐
3-(氨基甲基)-4,6-二甲基-2(1H)-吡啶酮盐酸盐
(4,6-二甲基-2-氧代-1,2-二氢吡啶-3-基)甲胺盐酸盐
3-(氨基甲基)-4,6-二甲基-1,2-二氢吡啶-2-酮盐酸盐
3 - (氨甲基)-4,6 - 二甲基-1H-吡啶-2 - 酮盐酸盐
3 - (氨甲基)-4,6 - 二甲基-1H-吡啶-2 - 酮盐酸盐
3-(AMinoMethyl)-4,6-diMethyl-1h-pyridin-2-one, HCl
3-(aminomethyl)-4,6-dimethylpyridin-2-ol hydrochloride
3-(Aminomethyl)-4,6-dimethylpyridin-2(1H)-one Hydrochlorid
3-(AMINOMETHYL)-4,6-DIMETHYL-1H-PYRIDIN-2-ONE HYDROCHLORIDE
3-(Aminomethyl)-4,6-dimethyl-2(1H)-pyridinone hydrochloride
3-(Aminomethyl)-4,6-dimethylpyridin-2(1H)-one dihydrochloride
2(1H)-Pyridinone, 3-(aMinoMethyl)-4,6-diMethyl-, hydrochloride
3-(Aminomethyl)-4,6-dimethyl-2(1H)-pyridinone hydrochloride (1:1)
(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methanaminium chloride
物理化学性质
制备方法
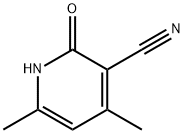
769-28-8

1173081-96-3
以2-氧代-4,6-二甲基-1,2-二氢吡啶-3-甲腈为原料合成3-(氨基甲基)-4,6-二甲基吡啶-2(1H)-酮盐酸盐的一般步骤:将4,6-二甲基-2-氧代-1,2-二氢吡啶-3-甲腈(10.0g,67.5mmol)溶于甲醇(1.50L)中,并在氮气保护下浓缩。随后,在氮气氛围下,向反应体系中加入浓盐酸(30mL)和10% Pd(OH)2催化剂(19g)。用氢气置换氮气,将反应混合物在室温及氢气氛围下搅拌26小时。反应完成后,再次用氮气置换氢气。将反应混合物通过硅藻土过滤,用甲醇洗涤滤饼,合并滤液并浓缩。将浓缩后的残余物用乙醇研磨,通过布氏漏斗收集固体产物,并在真空下干燥,得到3-(氨基甲基)-4,6-二甲基吡啶-2(1H)-酮盐酸盐,为白色固体(11.5g,收率90%)。1H NMR(400MHz,DMSO-d6):δ 11.86(宽峰,1H),5.98(单峰,1H),3.78(多重峰,2H),2.20(单峰,3H),2.16(单峰,3H)。
参考文献:
[1] Patent: US2014/107122, 2014, A1. Location in patent: Paragraph 0154; 0155
[2] Patent: WO2011/140324, 2011, A1. Location in patent: Page/Page column 143
[3] Patent: WO2011/140325, 2011, A1. Location in patent: Page/Page column 141-142
[4] Patent: WO2013/39988, 2013, A1. Location in patent: Page/Page column 50-51
[5] Patent: WO2013/173441, 2013, A2. Location in patent: Page/Page column 43; 44
