1196541-47-5
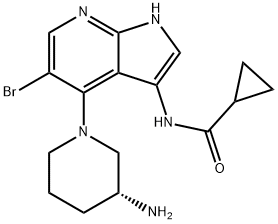 1196541-47-5 结构式
1196541-47-5 结构式
基本信息
(R)-N-(4-(3-氨基哌啶-1-基)-5-溴-1H-吡咯并[2,3-B]吡啶-3-基)环丙烷甲酰胺
RG-7741
ARRY-575
EOS-61380
ARRY-575, RG7741
GDC0575 (ARRY-575,RG7441)
GDC-0575 (ARRY-575, RG7741)
GDC0575
ARRY 575
RG7-441
GDC 0575
ARRY575
RG 7441
GDC-0575
ARRY-575
RG744
CYCLOPROPANECARBOXAMIDE, N-[4-[(3R)-3-AMINO-1-PIPERIDINYL]-5-BROMO-1H-PYRROLO[2,3-B]PYRIDIN-3-YL]
Cyclopropanecarboxamide, N-[4-[(3R)-3-amino-1-piperidinyl]-5-bromo-1H-pyrrolo[2,3-b]pyridin-3-yl]-
物理化学性质
DMSO:68.33(Max Conc. mg/mL);180.65(Max Conc. mM)
DMSO:PBS (pH 7.2) (1:5):0.16(Max Conc. mg/mL);0.42(Max Conc. mM)
Ethanol:3.0(Max Conc. mg/mL);7.93(Max Conc. mM)
制备方法
![(R)-tert-butyl1-(5-bromo-3-(cyclopropanecarboxamido)-1H-pyrrolo[2,3-b]pyridin-4-yl)piperidin-3-ylcarbamate](/CAS/20200611/GIF/1196508-04-9.gif)
1196508-04-9
![(R)-N-(4-(3-氨基哌啶-1-基)-5-溴-1H-吡咯并[2,3-B]吡啶-3-基)环丙烷甲酰胺](/CAS/20180703/GIF/1196541-47-5.gif)
1196541-47-5
(0010)向配备机械搅拌器、氮气/真空歧管、热电偶及冷凝器的1L惰性夹套反应器中,加入(R)-5-溴-4-(3-(叔丁氧基羰基氨基)哌啶-1-基)-3-硝基-1H-吡咯并[2,3-b]吡啶(1:1甲苯溶剂化物)(30.0g,1.00当量)及四氢呋喃(180mL,6.00mL/g)。随后,将4.5M哌嗪水溶液(42.4g溶于190mL水中)缓慢加入反应混合物中,同时保持温度在0℃。接着,加入硫酸(36.1mL,3.00当量),并在25℃下搅拌反应混合物2小时。反应完成后,加入15.0mL饱和盐水,分离水相。有机相在20℃下搅拌5分钟后,加入水(22.0mL),并在50℃下进行连续蒸馏。通过调节乙醇的进料速率以匹配蒸馏速率,直至加入总量为260mL的乙醇。随后,在50℃下1小时内加入水(340mL)。通过过滤分离所得固体,并用20%乙醇水溶液(2×60mL)洗涤。最后,在真空烘箱中50℃下干燥过夜,得到16.4g(78%校正产率)的(R)-5-溴-4-(3-氨基哌啶-1-基)-3-(环丙烷甲酰胺基)-1H-吡咯并[2,3-b]吡啶,为浅黄色固体。注:游离碱产物的1H NMR和13C NMR谱图信号较宽,因此所示谱图为游离碱转化为双HCl盐后的数据。1H NMR (300 MHz, DMSO-d6): δ 11.98 (br, 1H), 9.78 (s, 1H), 8.44 (br, 3H), 8.25 (s, 1H), 7.45 (d, J = 2.4 Hz, 1H), 3.57 (m, 1H), 3.43 (m, 1H), 3.41 (m, 1H), 3.28 (m, 1H), 3.14 (m, 1H), 2.15 (m, 1H), 1.90 (penta, J = 6.5 Hz, 1H), 1.81 (m, 1H), 1.72 (m, 1H), 1.52 (m, 1H), 0.83 (m, 4H)。13C NMR (75 MHz, DMSO-d6): δ 172.9, 149.5, 145.9, 145.1, 121.9, 114.2, 113.1, 107.8, 53.8, 51.1, 47.5, 28.6, 24.37, 14.7, 7.55, 7.45。HRMS-ESI (m/z): [M + H]+ 计算值 C16H21BrN5O, 378.0924; 实测值, 378.0912。
参考文献:
[1] Patent: WO2015/27090, 2015, A1. Location in patent: Paragraph 00100; 00101
[2] Organic Process Research and Development, 2018, vol. 22, # 3, p. 344 - 350
[3] Patent: WO2015/27092, 2015, A1. Location in patent: Paragraph 00100-00101
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/09/19 | S8526 | (R)-N-(4-(3-氨基哌啶-1-基)-5-溴-1H-吡咯并[2,3-B]吡啶-3-基)环丙烷甲酰胺 GDC-0575 (ARRY-575) | 1196541-47-5 | 5mg | 2023.92元 |
| 2025/09/19 | S8526 | (R)-N-(4-(3-氨基哌啶-1-基)-5-溴-1H-吡咯并[2,3-B]吡啶-3-基)环丙烷甲酰胺 GDC-0575 (ARRY-575) | 1196541-47-5 | 10mM(1mL in DMSO) | 2470元 |
| 2025/09/19 | S8526 | (R)-N-(4-(3-氨基哌啶-1-基)-5-溴-1H-吡咯并[2,3-B]吡啶-3-基)环丙烷甲酰胺 GDC-0575 (ARRY-575) | 1196541-47-5 | 25mg | 6118.42元 |