1197159-91-3
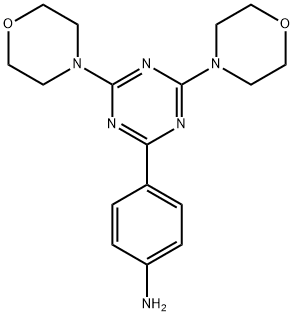 1197159-91-3 结构式
1197159-91-3 结构式
基本信息
4-(4,6-二吗啡啉-1,3,5-三嗪-2-基)苯胺
4-(4,6-二-4-吗啉-1,3,5-噻嗪-2-基)苯胺
4-(4,6-dimorpholino-1,3,5-triazin-2-yl)aniline
4-[4,6-Bis(4-morpholinyl)-1,3,5-triazin-2-yl]aniline
4-[4,6-bis(morpholin-4-yl)-1,3,5-triazin-2-yl]aniline
4-(4,6-di-4-morpholinyl-1,3,5-triazin-2-yl)benzenamine
Benzenamine, 4-(4,6-di-4-morpholinyl-1,3,5-triazin-2-yl)-
物理化学性质
制备方法
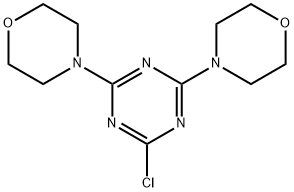
7597-22-0
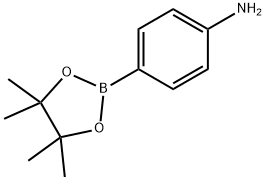
214360-73-3

1197159-91-3
以2-氯-4,6-二吗啉-1,3,5-三嗪(2.30 g,0.105 mol)和4-氨基苯硼酸频哪醇酯(25.7 g,0.117 mol)为原料,在碳酸钠(23 g,0.21 mol)和四(三苯基膦)钯(1 g,0.5 wt%)的存在下,于水(150 mL)和二甲氧基乙烷(DME,450 mL)的混合溶剂中加热回流5小时。反应完成后,将混合物冷却至室温,通过滤纸过滤。分离滤液中的有机层,用盐水洗涤后浓缩。将浓缩后的残余物溶解于二氯甲烷中,再次用盐水洗涤,经无水硫酸钠干燥后浓缩。所得固体用乙醚研磨,过滤后空气干燥,得到米色固体产物4-(4,6-二吗啉代-1,3,5-三嗪-2-基)苯胺(31.5 g,0.092 mol),收率88%。质谱分析显示目标化合物的分子离子峰为343.1(M + H)+。
参考文献:
[1] Patent: WO2010/96619, 2010, A1. Location in patent: Page/Page column 27-28
[2] Patent: US2009/291079, 2009, A1. Location in patent: Page/Page column 37
[3] Journal of Medicinal Chemistry, 2010, vol. 53, # 6, p. 2636 - 2645
[4] Patent: US2017/119778, 2017, A1. Location in patent: Paragraph 0929
[5] Patent: CN108191837, 2018, A. Location in patent: Paragraph 0179; 0180
