1201186-54-0
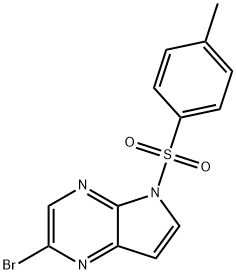 1201186-54-0 结构式
1201186-54-0 结构式
基本信息
N-甲苯磺酰基-5-溴-4,7-二氮杂吲哚
2-溴-5-对甲苯磺酰基-5H-吡咯并[2,3-B]吡嗪
2-溴-5-[(4-甲基苯基)磺酰基]-5H-吡咯并[2,3-B]吡嗪
2-BroMo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine
2-BROMO-5-(P-TOLYLSULFONYL)-5H-PYRROLO[2,3-B]PYRAZINE
2-bromo-5-(4-methylphenyl)sulfonylpyrrolo[2,3-b]pyrazine
2-bromo-5-(4-methylbenzenesulfonyl)-5H-pyrrolo[2,3-b]pyrazine
2-Bromo-5-[(4-methylphenyl)sulfonyl]-5H-pyrrolo[2,3-b]pyrazine
5H-Pyrrolo[2,3-b]pyrazine, 2-bromo-5-[(4-methylphenyl)sulfonyl]-
物理化学性质
制备方法
![3-[(三甲基硅基)乙炔基]-5-吡嗪-2-胺](/CAS2/GIF/875781-41-2.gif)
875781-41-2

98-59-9

1201186-54-0
在0℃条件下,向5-溴-3-[(三甲基硅基)炔基]吡嗪-2-胺(3.00g,11.1mmol)的DMF(60mL)溶液中分批加入NaH(60%分散于矿物油中,0.577g,14.4mmol)。反应15分钟后,加入对甲苯磺酰氯(2.75g,14.4mmol),随后将反应体系缓慢升温至室温。反应16小时后,将混合物倒入冰水(120mL)中,通过真空过滤收集沉淀。粗产物溶解于DCM(15mL)中,经硅胶柱色谱纯化,以DCM为洗脱剂。合并含目标产物的馏分,减压浓缩,得到2-溴-5-甲苯磺酰基-5H-吡咯并[2,3-b]吡嗪(2.16g,52%收率)。LC/MS分析(条件参见表2,方法d)显示:保留时间Rt=1.58分钟;质谱m/z:352/354(M+H)+。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 3, p. 693 - 698
[2] Bioorganic and Medicinal Chemistry Letters, 2015, vol. 25, # 20, p. 4399 - 4404
[3] Patent: US2009/312338, 2009, A1. Location in patent: Page/Page column 61
[4] Patent: WO2018/108125, 2018, A1. Location in patent: Paragraph 00587
[5] Patent: US2015/118229, 2015, A1. Location in patent: Paragraph 0184
