1202993-11-0
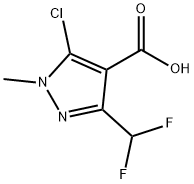 1202993-11-0 结构式
1202993-11-0 结构式
基本信息
5-氯-3-(二氟甲基)-1-甲基-1H-吡唑-4-羧酸
5-Chloro-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxyli...
5-Chloro-3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid
1H-Pyrazole-4-carboxylic acid, 5-chloro-3-(difluoroMethyl)-1-Methyl-
物理化学性质
制备方法
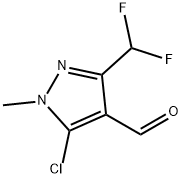
660845-30-7

1202993-11-0
在500 mL三口烧瓶中,将6.0 g(31 mmol)5-氯-3-(二氟甲基)-1-甲基-1H-吡唑-4-甲醛溶解于30 mL甲苯中。向该溶液中缓慢加入2.4 g(62 mmol)氢氧化钠溶于6 mL水的溶液,随后在温度控制在37℃以下的条件下,滴加103 mL 30%过氧化氢水溶液。反应混合物在50℃下搅拌反应7小时。反应完成后,冷却至室温,分离有机相和水相。用100 mL水萃取有机相,合并水相。用稀盐酸水溶液将合并的水相酸化至pH 2,析出白色沉淀。过滤收集沉淀,用20 mL水洗涤两次,干燥后得到3.2 g 5-氯-3-(二氟甲基)-1-甲基-1H-吡唑-4-羧酸,为白色固体。产物经1H NMR(400 MHz,DMSO-d6)表征:δ 3.78(s,3H),7.12(t,1H,JHF = 53.60 Hz),13.19(s,1H);IR(KBr)显示:1688 cm-1(C=O伸缩振动),2200-3200 cm-1宽峰(氢键振动)。
参考文献:
[1] Patent: EP2251331, 2010, A1. Location in patent: Page/Page column 24
[2] Patent: WO2011/151369, 2011, A1. Location in patent: Page/Page column 63
[3] Patent: WO2011/151370, 2011, A1. Location in patent: Page/Page column 90
[4] Patent: WO2012/52491, 2012, A2. Location in patent: Page/Page column 43
[5] Patent: WO2012/52490, 2012, A1. Location in patent: Page/Page column 68
