1226781-82-3
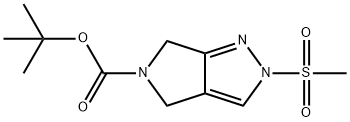 1226781-82-3 结构式
1226781-82-3 结构式
基本信息
奥格列汀杂质10
奥格列汀中间体IV
MK-3102 中间体IV
5-BOC-2-(甲磺酰基)-2,4,5,6-四氢吡咯并[3,4-C]吡唑
2-(甲基磺酰基)-2,6-二氢吡咯并[3,4-C]吡唑-5(4H)-甲酸叔丁酯
2-(甲基磺酰基)-2,6-二氢吡咯并[3,4-C]吡唑-5(4H)-羧酸叔丁酯
2-(甲基磺酰基)-4,6-二氢吡咯并[3,4-C]吡唑-5(2H)-羧酸叔丁酯
2-(甲基磺酰基)-4,6-二氢吡咯并[3,4-C]吡唑-5(2H)-甲酸叔丁酯
2-(甲基磺酰基)-2,6-二氢吡咯并[3,4-C]吡唑-5(4H)-羧酸叔丁酯 奥格列汀中间体
MK-3103 Intermediate IV
Omarigliptin Impurity 10
Omarigliptin Intermediate 3
5-Boc-2-(methylsulfonyl)-2,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole
tert-butyl 2-methanesulfonyl-2H,4H,5H,6H-pyrrolo[3,4-c]pyrazole-5-carboxylate
Pyrrolo[3,4-c]pyrazole, 2,4,5,6-tetrahydro-2-(methylsulfonyl)-, benzenesulfonate
tert-butyl 2-(Methylsulfonyl)-4,6-dihydropyrrolo[3,4-c]pyrazole-5(2H)-carboxylate
tert-butyl 2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazole-5(4H)-carboxylate
2-(Methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazole-5(4H)-carboxylic acid tert-butyl ester
物理化学性质
制备方法
1280210-79-8

124-63-0
![2-(甲基磺酰基)-2,6-二氢吡咯并[3,4-C]吡唑-5(4H)-羧酸叔丁酯](/CAS/20150408/GIF/1226781-82-3.gif)
1226781-82-3
将4,6-二氢吡咯并[3,4-c]吡唑-5(2H)-羧酸叔丁酯(3.5g,16.7mmol)溶解于四氢呋喃(35ml)中,冰浴冷却至0℃。在氮气保护下,缓慢加入氢化钠(1.0g,60%分散于矿物油中,25.4mmol),保持0℃搅拌反应30分钟。随后,逐滴加入甲基磺酰氯(2.9g,25.4mmol),继续在0℃下搅拌反应1小时。反应完成后,缓慢加入水(10ml)淬灭反应,用乙酸乙酯(50ml×2)萃取。合并有机相,用无水硫酸钠干燥,减压浓缩。粗产物通过硅胶柱色谱法纯化,洗脱剂为石油醚/乙酸乙酯(v/v=1:1),得到白色固体2-(甲基磺酰基)-4,6-二氢吡咯并[3,4-c]吡唑-5(2H)-甲酸叔丁酯(2.1g,产率44%)。
参考文献:
[1] Patent: EP3159344, 2017, A1. Location in patent: Paragraph 0096; 0132; 0133
[2] Patent: TW2017/8222, 2017, A. Location in patent: Page/Page column 54; 55
[3] Patent: TW2017/8223, 2017, A. Location in patent: Page/Page column 43
[4] Patent: WO2011/103256, 2011, A1. Location in patent: Page/Page column 55
[5] Patent: WO2011/146358, 2011, A1. Location in patent: Page/Page column 61
