124184-67-4
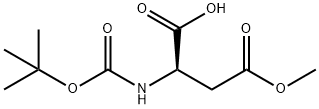 124184-67-4 结构式
124184-67-4 结构式
基本信息
BOC-D-天冬氨酸-Β-甲酯
N-BOC-D-天冬氨酸-4-甲酯
N-[(1,1-二甲基乙氧基)羰基]-D-天冬氨酸-4-甲酯
(R)-2-((叔丁氧基羰基)氨基)-4-甲氧基-4-氧代丁酸
(Tert-Butoxy)Carbonyl D-Asp(OMe)-OH
N-Boc-D-aspartic Acid 4-Methyl Ester
Boc-D-aspartic acid-beta-Methyl ester
(R)-2-((tert-Butoxycarbonyl)aMino)-4-Methoxy-4-oxobutanoic acid
D-Aspartic acid, N-[(1,1-dimethylethoxy)carbonyl]-, 4-methyl ester
(2R)-2-{[(tert-butoxy)carbonyl]amino}-4-methoxy-4-oxobutanoic acid
(2R)-4-methoxy-2-[(2-methylpropan-2-yl)oxycarbonylamino]-4-oxobutanoic acid
物理化学性质
制备方法

24424-99-5
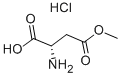
16856-13-6
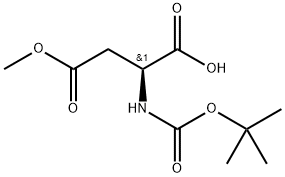
59768-74-0
在0℃下,将3.74g(20.5mmol)L-天冬氨酸-β-甲酯盐酸盐溶解于85mL二恶烷/H2O(2:1)混合溶剂中,向该溶液中加入2.21g(20.8mmol)Na2CO3。30分钟后,向反应混合物中再加入2.21g(20.8mmol)Na2CO3和5g(25.7mmol)二碳酸二叔丁酯(Boc2O),随后在室温下搅拌反应过夜。反应完成后,通过真空浓缩除去溶剂,将残余物倒入60mL冰水中。水层用2×25mL乙醚洗涤,然后用100mL 1M NaHSO4酸化至pH=3,之后水层用乙醚(3×75mL)萃取。合并有机层,用MgSO4干燥。过滤并蒸发溶剂后,残余物通过柱色谱法纯化,使用乙酸乙酯作为洗脱剂,得到目标产物2-(S)-(叔丁氧基羰基氨基)-(甲氧基羰基)丁酸,产率为75%。产物为白色固体,Rf值为0.50(乙酸乙酯),[α]D=+28.6(c=0.3; CHCl3)。IR光谱(cm-1):3429(NH),2979(CH),1714(C=O),1509,1438,1394,1367,1156,1057,1026,843,780,734。1H NMR(300MHz, CDCl3):δ(ppm)=1.42(s, 9H, CH3),2.82(dd, J=4.8,17.2Hz, 1H, CH2),3.02(dd, J=17.2,4.1Hz, 1H, CH2),3.69(s, 3H, OCH3),4.59-4.62(m, 1H, CHN),5.57(d, J=8.5Hz, 1H, NHBoc),10.8(sl, 1H, COOH)。13C NMR(75MHz, CDCl3):δ(ppm)=28.2(CH3),36.4(CH2),49.7(CHN),52.1(OCH3),80.5(C(CH3)3),155.6(COO),171.6(COO),175.8(COO)。元素分析(C10H17NO6)计算值:C 48.58, H 6.93, N 5.67;实测值:C 48.64, H 7.04, N 5.68。
参考文献:
[1] Heterocycles, 1997, vol. 46, # 1, p. 335 - 348
[2] Journal of Organic Chemistry, 1988, vol. 53, # 9, p. 1900 - 1903
[3] Chemical Communications, 2001, # 18, p. 1710 - 1711
[4] Patent: WO2013/30193, 2013, A1. Location in patent: Page/Page column 30; 31
[5] RSC Advances, 2015, vol. 5, # 88, p. 71868 - 71872
