124676-19-3
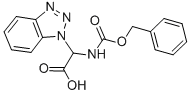 124676-19-3 结构式
124676-19-3 结构式
基本信息
2-(1H-苯并[D][1,2,3]噻唑-1-基)-2-(((苄氧基)羰基)氨基)乙酸
2-(1H-苯并[D][1,2,3]三唑-1-基)-2-(((苄氧基)羰基)氨基)乙酸
Benzotriazole-1H-ylbenzyloxyaminoaceticacid
BENZOTRIAZOL-1-YL-BENZYLOXYCARBONYLAMINO-ACETIC ACID
2-(1H-Benzotriazol-1-yl)-2-[(benzyloxycarbonyl)amino]acetic acid
α-[[(PhenylMethoxy)carbonyl]aMino]-1H-benzotriazole-1-acetic Acid
a-[[(Phenylmethoxy)carbonyl]amino]-1H-benzotriazole-1-acetic Acid
1H-1,2,3-benzotriazol-1-yl{[(benzyloxy)carbonyl]amino}acetic acid
1H-Benzotriazole-1-acetic acid, α-[[(phenylmethoxy)carbonyl]amino]-
alpha-[[(Phenylmethoxy)carbonyl]amino]-1H-benzotriazole-1-acetic acid
2-(1H-Benzo[d][1,2,3]triazol-1-yl)-2-(benzyloxycarbonylamino)acetic acid
物理化学性质
制备方法
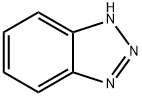
95-14-7
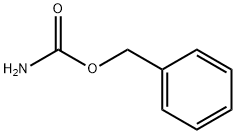
621-84-1
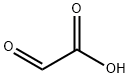
298-12-4

124676-19-3
在250毫升装有机械搅拌器的烧瓶中,依次加入2-氧代乙酸水合物(9.2克,0.1摩尔)、氨基甲酸苄酯(15.1克,0.1摩尔)和1H-苯并[d][1,2,3]三唑(9.2克,0.1摩尔),然后加入甲苯(300毫升)作为溶剂。将反应混合物在油浴中加热至120℃,并持续搅拌2小时。反应完成后,将混合物过滤,收集固体产物。用石油醚(3次)洗涤固体残余物,随后在真空条件下干燥,得到目标化合物2-(1H-苯并[d][1,2,3]三唑-1-基)-2-(((苄氧基)羰基)氨基)乙酸(28.6克,收率87%),为白色固体,无需进一步纯化即可用于后续反应。通过电喷雾电离质谱(ESI-MS)分析,测得分子离子峰m/z:327 [M + H]+。
参考文献:
[1] ACS Medicinal Chemistry Letters, 2015, vol. 6, # 5, p. 523 - 527
[2] Patent: WO2017/15449, 2017, A1. Location in patent: Page/Page column 51
[3] Patent: US2018/193352, 2018, A1. Location in patent: Paragraph 0205; 0206; 0207
[4] Journal of Organic Chemistry, 1990, vol. 55, # 4, p. 2206 - 2214
[5] Journal of the Chemical Society, Chemical Communications, 1989, # 6, p. 337 - 338
