1256359-15-5
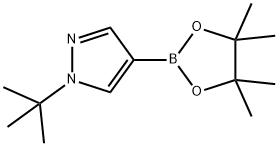 1256359-15-5 结构式
1256359-15-5 结构式
基本信息
1-(叔丁基)-4-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)吡唑
1-(叔-丁基)-4-(4,4,5,5-四甲基-1,3,2-二噁硼戊环-2-基)-1H-吡唑
1-tert-Butylpyrazole-4-boronic acid, pinacol ester
1-tert-Butylpyrazole-4-boronic acid, pinacol ester 97%
(1-(TERT-BUTYL)-1H-PYRAZOL-4-YL)BORONIC ACID PINACOL ESTER
1-tert-butyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole
1-tert-Butyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1H-Pyrazole, 1-(1,1-dimethylethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
1-tert-Butyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole1-tert-Butyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
物理化学性质
制备方法
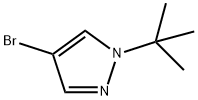
70951-85-8
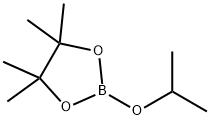
61676-62-8

1256359-15-5
向20 L四颈瓶中加入4-溴-1-叔丁基-1H-吡唑(1.15 kg,5.66 mol)和THF(9.2 L),将混合物冷却至-78°C至-85°C。在该温度下,逐滴加入正丁基锂(nBuLi,6.23 mol)。加完后,将反应液在相同温度下继续搅拌1小时,随后滴加异丙醇频哪醇硼酸酯(1.47 kg,7.92 mol)。反应液搅拌约3小时,直至反应完成(通过GC检测,原料残留<1.0%)。加入水(2.3 L)淬灭反应,随后用1 M HCl(3.45 kg)调节pH至8-9。水相用叔丁基甲基醚(TBME,3.45 L×2)萃取,合并的有机相依次用5% NaCl水溶液(3.45 L×2)和水(3.45 L)洗涤。蒸发有机相,得到粗产物。粗产物通过庚烷重结晶纯化,得到白色固体状纯产物(GC纯度99.7%),总产率为63.5%。注:进行了两次20 L规模的相同反应,合并后在庚烷中重结晶。
参考文献:
[1] Patent: WO2011/134971, 2011, A1. Location in patent: Page/Page column 16; 17; 73
[2] Patent: US2013/40984, 2013, A1. Location in patent: Paragraph 0484-0485
[3] Patent: WO2015/140054, 2015, A1. Location in patent: Page/Page column 48
[4] Patent: WO2017/42100, 2017, A1. Location in patent: Page/Page column 45; 46
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW02125635915504 | 1-(叔丁基)-4-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)吡唑 | 1256359-15-5 | 5G | 645元 |
| 2025/12/22 | XW02125635915503 | 1-(叔丁基)-4-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)吡唑 | 1256359-15-5 | 1G | 151元 |
| 2025/12/22 | XW02125635915502 | 1-(叔丁基)-4-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)吡唑 | 1256359-15-5 | 250MG | 48元 |


