1263774-42-0
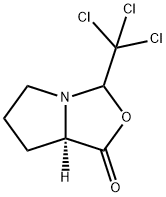 1263774-42-0 结构式
1263774-42-0 结构式
基本信息
(3R,7AR)-3-(三氯甲基)四氢-1H-吡咯并[1,2-C][1,3]氧杂-1-酮
(7aR)-Tetrahydro-3-(trichloromethyl)-1H,3H-pyrrolo[1,2-c]oxazol-1-one
1H,3H-Pyrrolo[1,2-c]oxazol-1-one, tetrahydro-3-(trichloromethyl)-, (7aR)-
(3R,7aR)-3-(Trichloromethyl)tetrahydro-1H-pyrrolo[1,2-c][1,3]oxaz ol-1-one
物理化学性质
制备方法
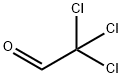
75-87-6
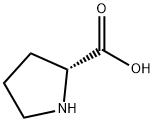
344-25-2
![(3R,7aR)-3-(Trichloromethyl)tetrahydro-1H-pyrrolo[1,2-c][1,3]oxaz ol-1-one](/CAS/20180703/GIF/1263774-42-0.gif)
1263774-42-0
以三氯乙醛和D-脯氨酸为原料合成(7AR)-3-(三氯甲基)四氢吡咯并[1,2-C]唑-1(3H)-酮的一般步骤:向D-脯氨酸(0.4 g,3.48 mmol)的乙腈(8 mL)溶液中加入三氯乙醛(0.68 mL,6.94 mmol),将反应混合物在室温下搅拌8小时。反应完成后,减压蒸发除去溶剂,残余物用乙醚研磨。过滤并干燥后,得到0.23 g (7AR)-3-(三氯甲基)四氢吡咯并[1,2-C]唑-1(3H)-酮(D48)。质谱(电喷雾正离子模式)m/z:244.0 [MH+],计算值C7H8Cl3NO2需242.96。1H NMR(400 MHz,CDCl3)δ(ppm):4.15(dd,J = 4.5, 8.6 Hz,1H),3.52-3.36(m,J = 7.0, 7.0, 10.5 Hz,1H),3.22-3.07(m,1H),2.33-2.20(m,1H),2.19-2.08(m,1H),1.96(quintet,J = 5.9, 12.1 Hz,1H),1.84-1.69(m,1H),1.61(br.s.,1H)。
参考文献:
[1] Patent: US2011/136778, 2011, A1. Location in patent: Page/Page column 54
[2] Patent: WO2013/4290, 2013, A1. Location in patent: Page/Page column 66
[3] Patent: US2014/243373, 2014, A1. Location in patent: Paragraph 0259-0261
[4] Patent: US2015/87626, 2015, A1. Location in patent: Paragraph 0504; 0505; 0506; 0507