126811-29-8
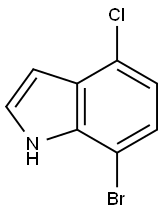 126811-29-8 结构式
126811-29-8 结构式
基本信息
7-Bromo-4-chloroindole
1H-Indole, 7-broMo-4-chloro-
物理化学性质
制备方法
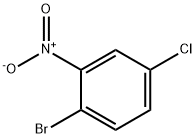
41513-04-6
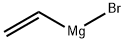
1826-67-1

126811-29-8
以2-溴-5-氯硝基苯和乙烯基溴化镁为原料合成4-氯-7-溴吲哚的一般步骤:将1.19 g(5 mmol)2-溴-5-氯硝基苯溶解于50 mL无水THF中。在氮气保护下,于-45℃缓慢滴加15 mL 1 M乙烯基溴化镁的THF溶液,控制反应温度不超过-40℃。滴加完毕后,将所得深色反应液于-40℃继续搅拌30分钟。反应完成后,在-40℃下用10 mL饱和氯化铵水溶液淬灭反应,随后用乙醚萃取两次。合并有机相,用饱和氯化钠水溶液洗涤一次,无水硫酸钠干燥,过滤并浓缩。残余物通过柱层析(100 g硅胶,洗脱剂为庚烷/乙酸乙酯19:1)纯化,得到562 mg(49%收率)7-溴-4-氯-1H-吲哚,为橙色油状物。质谱(EI)数据:m/z 229.0(80%),231.0(100%),233.0(30%)(M+)。
参考文献:
[1] Patent: US2006/30613, 2006, A1. Location in patent: Page/Page column 35
[2] Patent: WO2016/16370, 2016, A1. Location in patent: Page/Page column 39; 40
[3] Patent: US2014/274695, 2014, A1. Location in patent: Paragraph 0292; 0293
[4] Patent: KR101601357, 2016, B1. Location in patent: Paragraph 0328-0330
[5] Bioorganic and Medicinal Chemistry Letters, 2000, vol. 10, # 11, p. 1223 - 1226
