13196-10-6
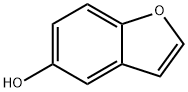 13196-10-6 结构式
13196-10-6 结构式
基本信息
5-苯并呋喃
苯并呋喃-5-醇
Benzofuran-5-ol
1-benzofuran-5-ol
Benzo[b]furan-5-ol
5-Hydroxybenzofuran
5-Hydroxybenzo[b]furan
物理化学性质
制备方法
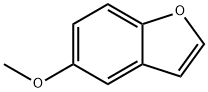
13391-28-1

13196-10-6
一般步骤:在冰浴冷却条件下,将5-甲氧基苯并呋喃(0.5 g,3.37 mmol)溶解于二氯甲烷(DCM,7 mL)中,缓慢加入三溴化硼(3.4 mL,3.37 mmol,1 M DCM溶液)。反应混合物在0℃下搅拌1小时后,再次加入等量的三溴化硼(3.4 mL)。随后,将反应混合物升至室温并继续搅拌2小时。通过薄层色谱(TLC)监测反应进程,确认反应完成后,将反应混合物缓慢倒入冰水中,并用碳酸钠(Na2CO3)调节pH至7。水相用二氯甲烷(DCM,2×)萃取,合并有机相后用饱和盐水洗涤,无水硫酸钠(Na2SO4)干燥,减压浓缩。所得浅棕色固体产物(0.36 g,收率79.6%)纯度满足后续反应要求,无需进一步纯化。产物结构经1H NMR(400 MHz,CD3OD)确认:δ 7.59(d,J = 2.0 Hz,1H),7.35(d,J = 9.2 Hz,1H),7.01(d,J = 2.4 Hz,1H),6.82(dd,J = 8.8 Hz,J = 2.8 Hz,1H),6.67(m,1H),4.73(s,1H)。
参考文献:
[1] Chemical Communications, 2016, vol. 52, # 16, p. 3348 - 3351
[2] Synthetic Communications, 2006, vol. 36, # 14, p. 1983 - 1990
[3] Patent: WO2007/61458, 2007, A2. Location in patent: Page/Page column 43; 91
[4] Patent: WO2017/87965, 2017, A1. Location in patent: Page/Page column 23
[5] Patent: WO2018/13774, 2018, A1. Location in patent: Page/Page column 379; 380
