133852-23-0
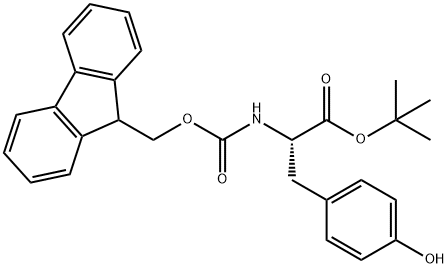 133852-23-0 结构式
133852-23-0 结构式
基本信息
芴甲氧羰基-L-酪氨酸-叔丁酯
N-[芴甲氧羰基]-L-酪氨酸叔丁酯
(S)-2-((((9H-芴-9-基)甲氧基)羰基)氨基)-3-(4-羟基苯基)丙酸叔丁酯
Fmoc-L-Tyr-OtBu
N-Fmoc-L-tyrosine tert-butyl ester
"L-Tyrosine (Tbu)-OH-13C9,15N, Fmoc"
(9H-Fluoren-9-yl)MethOxy]Carbonyl Tyr-OtBu
tert-butyl (((9H-fluoren-9-yl)methoxy)carbonyl)-L-tyrosinate
N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-tyrosine 1,1-dimethylethyl ester
L-Tyrosine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-, 1,1-dimethylethyl ester
tert-butyl (2S)-2-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)-3-(4-hydroxyphenyl)propanoate
物理化学性质
制备方法
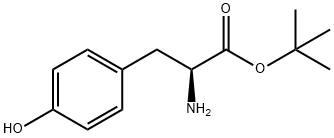
16874-12-7
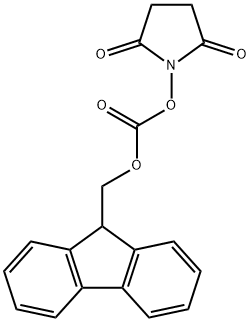
82911-69-1
![N-[芴甲氧羰基]-L-酪氨酸叔丁酯](/CAS/20180713/GIF/133852-23-0.gif)
133852-23-0
一般步骤:将L-酪氨酸叔丁酯(1.00 g,4.21 mmol)和碳酸氢钠(354 mg,4.21 mmol)悬浮于1,4-二氧六环/水(1:1,20 mL)中,搅拌。向该悬浮液中加入9-芴甲基-N-琥珀酰亚胺基碳酸酯(1.42 g,4.21 mmol),所得混合物在室温下搅拌18小时。反应完成后,将溶剂减压浓缩至约10 mL。加入冷的1N盐酸(50 mL),用乙酸乙酯(3×50 mL)萃取产物。合并有机相,依次用水和饱和氯化钠溶液洗涤,无水硫酸钠干燥。过滤后,减压浓缩滤液,得到无色固体产物83(1.94 g,100%),无需进一步纯化即可用于下一步反应。产物经1H NMR(400 MHz,CDCl3)确认:δ 1.43(s,9H),2.97-3.06(m,2H),4.21(bt,J = 7.1 Hz,1H),4.33(dd,J = 7.1,10.5 Hz,1H),4.41-4.53(m,2H),5.01(bs,1H),5.30(d,J = 8.2 Hz,1H),6.73(d,J = 8.5 Hz,2H),7.00(d,J = 8.5 Hz,2H),7.31(t,J = 7.5 Hz,2H),7.40(t,J = 7.5 Hz,2H),7.57(dd,J = 3.3,7.3 Hz,2H),7.76(d,J = 7.5 Hz,2H)。
参考文献:
[1] Patent: WO2010/19511, 2010, A2. Location in patent: Page/Page column 125-126
[2] Chemical Communications, 2011, vol. 47, # 15, p. 4439 - 4441
[3] Patent: CN104274839, 2017, B. Location in patent: Paragraph 0036; 0038; 0040; 0041
[4] Angewandte Chemie - International Edition, 2009, vol. 48, # 11, p. 2024 - 2026
[5] Tetrahedron Letters, 1995, vol. 36, # 27, p. 4733 - 4736