135481-57-1
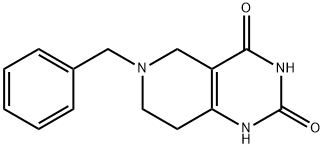 135481-57-1 结构式
135481-57-1 结构式
基本信息
6-苯甲基-5,6,7,8-四氢-1H-哌啶并[4,3-D]2,4-二嘧啶酮
6-苄基-5,6,7,8-四氢吡啶并[4,3-D]嘧啶-2,4(1H,3H)-二酮
5,6,7,8-四氢-6(苯基甲基)吡啶并[4,3-D]嘧啶-2,4(1H,3H)-二酮
6-Benzyl-5,6,7,8-tetrahyd...
6-Benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine-2,4(1H,3H)
6-BENZYL-5,6,7,8-TETRAHYDRO-1H-PYRIDO[4,3-D]PYRIMIDINE-2,4-DIONE
6-Benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyriMidin-2,4(1H,3H)-dione
6-benzyl-1H,2H,3H,4H,5H,6H,7H,8H-pyrido[4,3-d]pyriMidine-2,4-dione
6-Benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine-2,4(1H,3H)-dione
6-(phenylmethyl)-1,5,7,8-tetrahydropyrido[4,3-d]pyrimidine-2,4-dione
6-Benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine-2,4(1H,3H)-dione 95%
5,6,7,8-Tetrahydro-6-(phenylmethyl)pyrido[4,3-d]pyrimidine-2,4(1H,3H)-dione
物理化学性质
制备方法
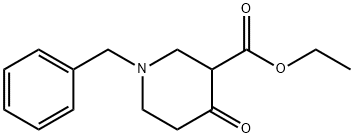
41276-30-6
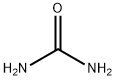
57-13-6
![6-苯甲基-5,6,7,8-四氢-1H-哌啶并[4,3-D]2,4-二嘧啶酮](/CAS/GIF/135481-57-1.gif)
135481-57-1
一般步骤:将1-苄基-4-哌啶酮-3-羧酸乙酯(10.0 g,33.5 mmol)溶解于无水乙醇(150.0 mL)中,随后依次加入尿素(10.0 g,167.0 mmol)和甲醇钠(22.7 g,118.0 mmol)。将反应混合物在加热回流条件下搅拌反应24小时。反应完成后,将混合物冷却至0℃,过滤收集析出的固体。将所得固体悬浮于去离子水中,缓慢加入6.0 mol/L盐酸溶液调节pH至6.0。室温下继续搅拌1小时后,过滤分离晶体,真空干燥,得到6-苯甲基-5,6,7,8-四氢-1H-哌啶并[4,3-D]2,4-二嘧啶酮(6.5 g,收率75%),产物无需进一步纯化即可用于后续反应。薄层色谱(TLC)分析:Rf = 0.1(展开剂:乙酸乙酯/甲醇=5:1);熔点:293℃;1H NMR(300 MHz,CDCl3,δ ppm):2.43-2.45(m,2H),2.51-2.52(m,2H),3.01(s,2H),3.53(s,2H),7.23-7.41(m,5H),10.21(s,1H),11.01(s,1H)。
参考文献:
[1] European Journal of Medicinal Chemistry, 2014, vol. 79, p. 399 - 412
[2] Journal of Medicinal Chemistry, 2016, vol. 59, # 23, p. 10498 - 10519
[3] Patent: EP1537879, 2005, A1. Location in patent: Page/Page column 61
[4] Patent: EP1547616, 2005, A1. Location in patent: Page/Page column 65
