13669-05-1
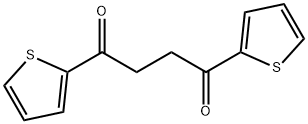 13669-05-1 结构式
13669-05-1 结构式
基本信息
1,4-二(2-噻吩基)-1,4-丁二酮
1,4-二噻吩-2-基 - 丁烷-1,4-二酮
1,4-DI(2-THIENYL)-1,4-BUTANEDIONE 1,4-双(2-噻吩基)-1,4-丁二酮
1,4-Di(2-thienyl)-1,4-butanedione 1,4-双(2-噻吩基)-1,4-丁二酮
1,4-di(2'-thienyl)-1,4-butadione
1,4-Di(2-thienyl)-1,4-butanedione
1,4-Di(2-thienyl)butane-1,4-dione
1,4-Butanedione,1,4-di-2-thienyl-
1,4-Di(2-thienyl)-1,4-butanedione>
1,4-di(thiophen-2-yl)butane-1,4-dione
1,4-di(thiophen-3-yl)butane-1,4-dione
1,4-bis(thiophen-2-yl)butane-1,4-dione
物理化学性质
制备方法
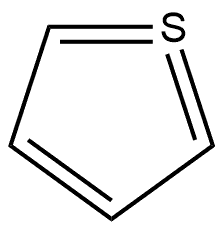
188290-36-0
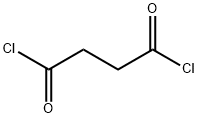
543-20-4

13669-05-1
以噻吩(9.6 mL,0.12 mol)和丁二酰氯(5.4 mL,0.05 mol)为原料,在100 mL二氯甲烷中制备含有AlCl3(16.2 g,0.12 mol)的悬浮液。将噻吩和丁二酰氯的混合溶液缓慢滴加到上述悬浮液中。反应混合物在40℃下搅拌4小时。反应完成后,将混合物倒入由100 g冰和10 mL盐酸组成的混合液中,继续搅拌30分钟。用二氯甲烷萃取反应混合物,依次用NaHCO3溶液和蒸馏水洗涤有机层,然后用无水MgSO4干燥。减压蒸发除去溶剂,所得固体残余物通过柱色谱法纯化,得到白色固体产物1,4-二(2-噻吩基)-1,4-丁二酮,产率为76%。产物结构通过1H NMR(500 MHz, CDCl3, 25℃, TMS, δ):7.84(dd, J = 3.8, 1.1 Hz, 2H),7.67(dd, J = 5.0, 1.1 Hz, 2H),7.17(dd, J = 4.9, 3.8 Hz, 2H),3.42(s, 4H)和MALDI-TOF MS(m/z):251.1 [M+ + H]进行确认。
参考文献:
[1] Asian Journal of Chemistry, 2013, vol. 25, # 15, p. 8772 - 8774
[2] Bioorganic and Medicinal Chemistry Letters, 1998, vol. 8, # 19, p. 2695 - 2698
[3] Journal of Materials Chemistry, 2000, vol. 10, # 1, p. 107 - 114
[4] Electrochimica Acta, 2017, vol. 229, p. 271 - 280
[5] Journal of Materials Chemistry, 2011, vol. 21, # 33, p. 12337 - 12343
