13726-21-1
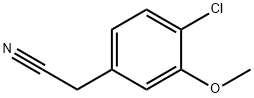 13726-21-1 结构式
13726-21-1 结构式
基本信息
4-氯-3-甲氧基苯基乙腈
2-(4-氯-3-甲氧基苯基)乙腈
2-(4-Chloro-3-methoxyphenyl)
(4-CHLORO-3-METHOXYPHENYL)ACETONITRILE
2-(4-Chloro-3-Methoxyphenyl)acetonitrile
Benzeneacetonitrile, 4-chloro-3-methoxy-
2-(4-Chloro-3-methoxyphenyl)acetonitrilev
物理化学性质
制备方法
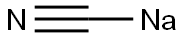
143-33-9
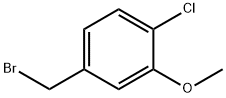
103347-14-4

13726-21-1
向4-(溴甲基)-1-氯-2-甲氧基苯(68.5 g,0.290 mol)的90%乙醇(500 mL)溶液中加入氰化钠(28.5 g,0.580 mol)。将反应混合物在60℃下搅拌过夜。反应完成后,减压蒸发除去乙醇,将残余物溶解于水中。用乙酸乙酯(300 mL × 3)萃取水相。合并有机层,用饱和食盐水洗涤,无水硫酸钠干燥。通过硅胶柱色谱法(洗脱剂:石油醚/乙酸乙酯 = 30:1)纯化,得到4-氯-3-甲氧基苯基乙腈(25 g,收率48%)。1H NMR (400 MHz, CDCl3) δ 7.36 (d, J = 8 Hz, 1H), 6.88-6.84 (m, 2H), 3.92 (s, 3H), 3.74 (s, 2H)。13C NMR (100 MHz, CDCl3) δ 155.4, 130.8, 129.7, 122.4, 120.7, 117.5, 111.5, 56.2, 23.5。
参考文献:
[1] Patent: US2007/244159, 2007, A1. Location in patent: Page/Page column 91
[2] Patent: US2005/239881, 2005, A1. Location in patent: Page/Page column 27
[3] Patent: WO2007/39736, 2007, A1. Location in patent: Page/Page column 28
[4] Patent: US2008/293775, 2008, A1. Location in patent: Page/Page column 22
[5] Patent: US2004/192704, 2004, A1. Location in patent: Page 23