137618-48-5
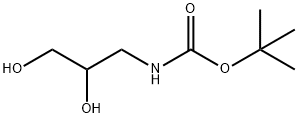 137618-48-5 结构式
137618-48-5 结构式
基本信息
N-BOC-3-氨基-1,2-丙二醇
(2,3-二羟基丙基)氨基甲酸叔丁酯
N-(2,3-二羟基丙基)氨基甲酸叔丁酯
BOC-(RS)-3-氨基-1,2-丙二醇
3-Boc-amino-1,2-propanediol
N-Boc-3-amino-1,2-propanediol
tert-Butyl (2,3-dihydroxypropyl)
BOC-(RS)-3-AMINO-1,2-PROPANEDIOL
tert-Butyl (2,3-dihydroxypropyl)carbaMate
ert-butylN-(2,3-dihydroxypropyl)carbamate
TERT-BUTYL N-(2,3-DIHYDROXYPROPYL)CARBAMATE
tert-Butyl N-(2,3-dihydroxypropyl)carbamate 97%
2-Methyl-2-Propanyl (2,3-Dihydroxypropyl)Carbamate
物理化学性质
制备方法
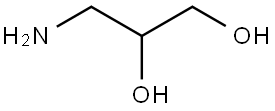
616-30-8

24424-99-5

137618-48-5
一般步骤:将3-氨基-1,2-丙二醇(3g,32.93mmol,1.0当量)溶解于20mL叔丁醇和20mL 1M NaOH的混合溶液中。在冰浴冷却条件下,缓慢加入含有二碳酸二叔丁酯(7.19g,32.93mmol,1.0当量)的20mL叔丁醇溶液。反应混合物在室温下搅拌5小时。通过薄层色谱(TLC)监测反应进程,确认原料完全消耗后,将反应液在50℃水浴中减压浓缩。随后,用1M HCl溶液中和反应混合物。中和后的溶液用乙酸乙酯和水进行液-液萃取,重复三次。合并有机层,用饱和氯化钠水溶液洗涤,无水硫酸镁干燥。干燥后的有机相在40℃水浴中减压浓缩,得到6.3g N-(2,3-二羟基丙基)氨基甲酸叔丁酯(产率:定量)。产物结构通过1H-NMR确认:1H-NMR(500MHz,CDCl3)δ1.45(9H,s),2.68-2.71(1H,br),2.75-2.81(1H,br),3.20(2H,dd),3.63(2H,dd),3.71(1H,m),4.91(1H,br)。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 2, p. 623 - 627
[2] Patent: US2016/151506, 2016, A1. Location in patent: Paragraph 0308-0312
[3] Canadian Journal of Chemistry, 1997, vol. 75, # 7, p. 942 - 948
[4] Patent: US2006/69156, 2006, A1. Location in patent: Page/Page column 26
[5] Patent: US2006/154984, 2006, A1. Location in patent: Page/Page column 41
