1378388-20-5
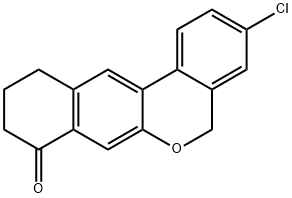 1378388-20-5 结构式
1378388-20-5 结构式
基本信息
GS-5816中间体-氯代
3-氯-10,11-二氢-5H-二苯并[C,G]色满-8(9H)-酮
3-氯-10,11-二氢-5H-苯并[D]萘并[2,3-B]吡喃-8(9H)-酮
3-chloro-10,11-dihydro-5H-dibenzo[c,g]chromen-8(9H)-one
3-Chloro-10,11-dihydro-5H,9H-6-oxa-benzo[a]anthracen-8-one
3-Chloro-10,11-dihydro-5H-benzo[d]naphtho[2,3-b]pyran-8(9H)-one
3-Chloro-10,11-dihydro-5H-benzo[d]naphtho[2,3-b]pyran-8(9H)-...
5H-Benzo[d]naphtho[2,3-b]pyran-8(9H)-one, 3-chloro-10,11-dihydro-
制备方法
![7-[(2-溴-5-氯苯基)甲氧基]-3,4-二氢-1(2H)-萘酮](/CAS/20150408/GIF/1378388-19-2.gif)
1378388-19-2

1378388-20-5
以7-[(2-溴-5-氯苯基)甲氧基]-3,4-二氢-1(2H)-萘酮为原料合成3-氯-10,11-二氢-5H-二苯并[c,g]色满-8(9H)-酮的一般步骤:向1L烧瓶中加入新戊酸钯(II)(1.18g,3.8mmol)、三(4-氟苯基)膦(1.20g,3.8mmol)、新戊酸(2.33g,22.8mmol)和碳酸钾(31.8g,228mmol),随后加入7-[(2-溴-5-氯苯基)甲氧基]-3,4-二氢-1(2H)-萘酮(27.8g,76.2mmol)的二甲基乙酰胺(380mL)溶液。将烧瓶抽真空并用氩气回填,此过程重复5次。在氩气保护下,将反应混合物于60℃搅拌24小时。反应完成后,冷却至室温,用甲基叔丁基醚(MTBE)和水稀释。将所得两相混合物搅拌3小时,通过硅藻土过滤,并用MTBE冲洗。分离滤液的有机层,依次用水洗涤两次和盐水洗涤一次。有机层用硫酸镁干燥,过滤后浓缩,通过快速柱色谱法(己烷/二氯甲烷)纯化,得到3-氯-10,11-二氢-5H-二苯并[c,g]色满-8(9H)-酮(14.4g,收率67%),为灰白色固体。
参考文献:
[1] Patent: WO2013/75029, 2013, A1. Location in patent: Page/Page column 53-55
[2] Patent: US2013/309196, 2013, A1. Location in patent: Paragraph 0206
[3] Patent: US2014/178336, 2014, A1. Location in patent: Paragraph 0235
[4] Patent: US2015/361073, 2015, A1. Location in patent: Paragraph 0438
[5] Patent: CN106916134, 2017, A. Location in patent: Paragraph 0013; 0023
