138227-63-1
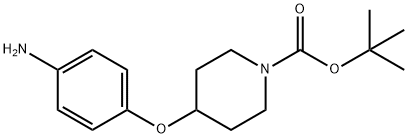 138227-63-1 结构式
138227-63-1 结构式
基本信息
1-BOC-4-(4-氨基苯氧基)哌啶
4-(4-氨基苯氧基)-1-哌啶甲酸叔丁酯
4-(4-氨基苯氧基)-1-哌啶羧酸-1,1-二甲基乙酯
N-Boc-4-(4-aminophenoxy)piperidine
1-BOC-4-(4-AMINO-PHENOXY)-PIPERIDINE
1N-BOC 4-(4'-AMINOPHENOXY) PIPERIDINE
tert-Butyl 4-(4-aminophenoxy)piperidine-1-carboxylate
4-{[1-(tert-Butoxycarbonyl)piperidin-4-yl]oxy}aniline
tert-Butyl 4-(4-aminophenoxy)-1-piperidinecarboxylate
4-{[1-(tert-Butoxycarbonyl)piperidin-4-yl]oxy}aniline 98%
4-(4-aminophenoxy)-1-piperidinecarboxylic acid tert-butyl ester
4-(4-AMINO-PHENOXY)-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
物理化学性质
制备方法
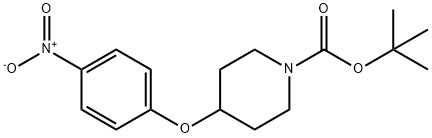
138227-62-0

138227-63-1
以4-(4-硝基苯氧基)哌啶-1-羧酸叔丁酯为原料合成1-Boc-4-(4-氨基苯氧基)哌啶的一般步骤如下:将参考实施例105中得到的4-(1-叔丁氧基羰基哌啶-4-基氧基)硝基苯(11.9 g)溶于甲醇(100 mL)中,加入钯碳催化剂(1.9 g)。将反应混合物在室温下于氢气氛围中搅拌4小时。反应完成后,通过过滤去除催化剂,滤液经减压浓缩。所得粗产物通过硅胶柱色谱法纯化,洗脱剂为体积比1:1的己烷与乙酸乙酯混合溶剂,最终得到1-Boc-4-(4-氨基苯氧基)哌啶(10.7 g,产率99%),为淡红色固体。1H NMR(400 MHz,CDCl3)δ ppm:1.46(9H,s),1.71(2H,m),1.87(2H,m),3.27(2H,m),3.71(2H,m),4.26(1H,m),6.63(2H,d,J = 8.5 Hz),6.76(2H,d,J = 8.5 Hz)。
参考文献:
[1] Chemical and Pharmaceutical Bulletin, 2008, vol. 56, # 6, p. 758 - 770
[2] Patent: EP1375482, 2004, A1. Location in patent: Page 159
[3] Bioorganic and medicinal chemistry, 2002, vol. 10, # 5, p. 1509 - 1523
[4] European Journal of Medicinal Chemistry, 2016, vol. 117, p. 1 - 7
[5] Patent: WO2015/92431, 2015, A1. Location in patent: Page/Page column 205; 206
