141699-58-3
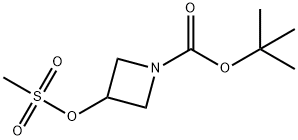 141699-58-3 结构式
141699-58-3 结构式
基本信息
1-BOC-3-OMS-氮杂环丁烷
1-BOC-3-(甲磺酰氧基)吖丁啶
N-BOC-3-甲基磺酰氧基氮杂环丁烷
N-BOC-3-(甲磺酰氧基)氮杂环丁烷
1-BOC-3-(甲烷磺酰氧基)氮杂环丁烷
3-甲基磺酰氧基氮杂环丁烷-1-羧酸叔丁酯
3-甲基磺酰氧基氮杂环丁烷-1-甲酸叔丁酯
3-甲基磺酰氧基-1-氮杂环丁烷羧酸叔丁酯
3-(甲磺酰氧基)氮杂环丁烷-1-羧酸叔丁酯
N-Boc-3-methanesulfonyloxyazetidine
1-Boc-3-methanesulfonyloxy-azetidine
N-Boc 3-(Methylsulfonyloxy)azetidine
1-N-BOC-3-METHANESULFONYLOXYAZETIDINE
1-(tert-Butoxycarbonyl)-3-(mesyloxy)azetidine
tert-butyl 3-methylsulfonyloxyazetidine-1-carboxylate
1-(tert-butoxycarbonyl)azetidine-3-yl-methanesulfonate
tert-Butyl 3-[(methylsulfonyl)oxy]azetane-1-carboxylate
1-(Tert-butoxycarbonyl)-3-(methanesulfonyloxy)azetidine
物理化学性质
制备方法

124-63-0
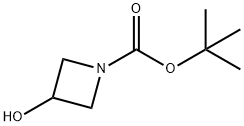
141699-55-0

141699-58-3
制备实施例26-2:3-((甲基磺酰基)氧基)氮杂环丁烷-1-羧酸叔丁酯 在氮气氛下,将甲基磺酰氯(2.57 mL,33.3 mmol)和三乙胺(11.6 mL,83.1 mmol)依次加入含有N-Boc-3-羟基氮杂环丁烷(4.8 g,27.7 mmol)的四氢呋喃(100 mL)溶液中。反应混合物在室温下搅拌2小时。反应完成后,向反应液中加入饱和碳酸氢钠水溶液,并用乙酸乙酯稀释混合物。分离有机层,用饱和盐水洗涤,随后用无水硫酸钠干燥。过滤除去干燥剂,滤液经真空浓缩。残余物通过硅胶柱色谱法(洗脱剂比例:正庚烷/乙酸乙酯=9:1至1:1)纯化,定量得到N-Boc-3-甲基磺酰氧基氮杂环丁烷。 1H-NMR (CDCl3) δ (ppm): 1.45 (9H, s), 3.07 (3H, s), 4.03-4.18 (2H, m), 4.22-4.36 (2H, m), 5.12-5.27 (1H, m)。
参考文献:
[1] Patent: US2014/235614, 2014, A1. Location in patent: Paragraph 0570; 0571; 0572
[2] Patent: EP1340757, 2003, A1
[3] Journal of Medicinal Chemistry, 2014, vol. 57, # 18, p. 7731 - 7757
[4] Patent: WO2018/102419, 2018, A1. Location in patent: Paragraph 0344; 0345; 0346; 0347
[5] Patent: US2012/10182, 2012, A1. Location in patent: Page/Page column 40
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0214169958306 | N-Boc-3-甲基磺酰氧基氮杂环丁烷 1-Boc-3-Methanesulfonyloxyazetidine | 141699-58-3 | 500g | 3417元 |
| 2025/12/22 | XW0214169958305 | N-Boc-3-甲基磺酰氧基氮杂环丁烷 1-Boc-3-Methanesulfonyloxyazetidine | 141699-58-3 | 100g | 705元 |
| 2025/12/22 | XW0214169958304 | N-Boc-3-甲基磺酰氧基氮杂环丁烷 1-Boc-3-Methanesulfonyloxyazetidine | 141699-58-3 | 25g | 195元 |
