142752-12-3
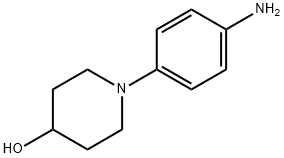 142752-12-3 结构式
142752-12-3 结构式
基本信息
1-(4-氨基苯基)-4-羟基哌啶
1-(4-aminophenyl)piperidin-4-ol
4-Piperidinol, 1-(4-aminophenyl)-
1-(4-AMINOPHENYL)-4-HYDROXYPIPERIDINE
物理化学性质
制备方法
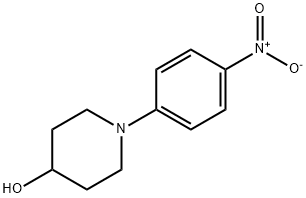
79421-45-7

142752-12-3
以1-(4-硝基苯基)哌啶-4-醇为原料合成1-(4-氨基苯基)-4-羟基哌啶的一般步骤如下:将1-(4-硝基苯基)哌啶-4-醇(1.50g,6.75mmol)溶解于甲醇(25ml)中,加入10%钯-碳催化剂(150mg)。将反应体系置换为氢气氛围,于室温下搅拌反应2小时。反应完成后,通过硅藻土过滤反应混合物,并用甲醇洗涤滤饼。合并滤液与洗涤液,通过减压蒸馏除去溶剂,得到目标产物1-(4-氨基苯基)-4-羟基哌啶(1.28g,收率99%)。产物结构经1H-NMR(CDCl3/TMS)确认,化学位移δppm如下:1.70-1.80(m,2H),2.07(br,2H),2.84(br,2H),3.37(br,3H),3.85(br,1H),6.65(d,J=8.4Hz,2H),6.91(br,2H)。
参考文献:
[1] Patent: EP1988077, 2008, A1. Location in patent: Page/Page column 64-65
[2] Patent: WO2012/59932, 2012, A1. Location in patent: Page/Page column 112
[3] Patent: US2011/319413, 2011, A1. Location in patent: Page/Page column 11
[4] Patent: EP1775298, 2007, A1. Location in patent: Page/Page column 39
[5] Patent: US2007/32478, 2007, A1. Location in patent: Page/Page column 147
