1427587-32-3
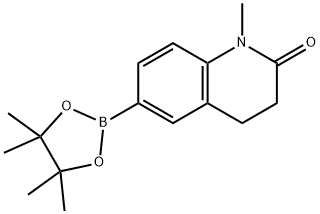 1427587-32-3 结构式
1427587-32-3 结构式
基本信息
1-甲基-2-氧代-1,2,3,4-四氢喹啉-6-硼酸频哪醇酯
1-甲基-6-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)-3,4-二氢喹啉-2(1H)-酮
1-Methyl-1,2,3,4-tetrahydroquinolin-2-one-6-boronic acid pinacol ester
1-Methyl-2-oxo-1,2,3,4-tetrahydroquinoline-6-boronic Acid Pinacol Ester
(1-METHYL-2-OXO-1,2,3,4-TETRAHYDROQUINOLIN-6-YL)BORONIC ACID PINACOL ESTER
1-methyl-6-(tetramethyl-1,3,2-dioxaborolan-2-yl)-1,2,3,4-tetrahydroquinolin-2-one
1-methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydroquinolin-2-one
1-Methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydroquinolin-2(1H)-one
2(1H)-Quinolinone, 3,4-dihydro-1-methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
物理化学性质
制备方法
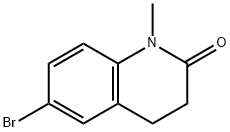
1092523-03-9

73183-34-3

1427587-32-3
向干燥的反应烧瓶中加入6-溴-1-甲基-3,4-二氢喹啉-2(1H)-酮(3g,12.5mmol)、联硼酸频那醇酯(3.81g,15.0mmol)、乙酸钾(3.68g,37.5mmol)及二恶烷(48mL)。在氩气保护下,向反应体系中加入二氯[1,1'-双(二苯基膦基)二茂铁]钯(II)二氯甲烷络合物(457mg,0.625mmol)。将反应混合物加热至80℃并搅拌过夜。反应完成后,用乙酸乙酯稀释反应混合物,通过硅藻土过滤,并用乙酸乙酯(2×150mL)洗涤滤饼。合并有机相,用饱和食盐水洗涤,无水硫酸钠干燥,过滤后减压浓缩。残余物通过硅胶柱色谱纯化,采用0至40%乙酸乙酯-庚烷梯度洗脱,得到目标产物1-甲基-6-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)-3,4-二氢喹啉-2(1H)-酮(2.63g,收率73%),为灰白色固体。质谱(MS):288.0(M+H+)。
参考文献:
[1] Patent: US2013/72679, 2013, A1. Location in patent: Paragraph 0545; 0546
[2] Patent: WO2013/37779, 2013, A1. Location in patent: Page/Page column 74; 75
[3] Patent: WO2013/41591, 2013, A1. Location in patent: Page/Page column 65
[4] Patent: WO2014/139981, 2014, A1. Location in patent: Page/Page column 24; 25
[5] Patent: WO2016/66662, 2016, A1. Location in patent: Page/Page column 31
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW02142758732305 | 1-甲基-6-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)-3,4-二氢喹啉-2(1H)-酮 | 1427587-32-3 | 10G | 2090元 |
| 2025/12/22 | XW02142758732304 | 1-甲基-6-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)-3,4-二氢喹啉-2(1H)-酮 | 1427587-32-3 | 5G | 1131元 |
| 2025/12/22 | XW02142758732303 | 1-甲基-6-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)-3,4-二氢喹啉-2(1H)-酮 | 1427587-32-3 | 1G | 290元 |