145589-03-3
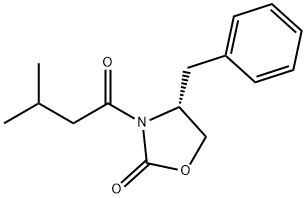 145589-03-3 结构式
145589-03-3 结构式
基本信息
(R)-3-(3-甲基丁酰)-4-苄基-2-噁唑烷酮
(R)-3-(3-甲基丁酰)-4-苄基-2-恶唑烷酮
(4R)-3-(3-甲基丁酰)-4-苄基-2-恶唑烷酮
(4R)-4-苄基-3-(3-甲基-丁酰基)-噁唑-2-酮
(R)-3-(3-甲基-1-氧代丁基)-4-苄基-2-噁唑烷酮
(R)-3-(3-甲基丁酰)-4-苄基-2-恶唑烷酮(阿利克伦中间体)
(4R)-4-Benzyl-3-isovaleryloxazolidin-2-one
3-isovaleroyl-4(R)-benzyl-oxazolidin-2-one
4R-Benzyl-3-(3-Methylbutyryl)oxazolidin-2-one
(R)-3-(3-methylbutanoyl)-4-benzyloxazolidin-2-one
(R)-4-benzyl-3-(3-Methylbutanoyl)oxazolidin-2-one
(4R)-4-benzyl-3-(3-Methylbutanoyl)-1,3-oxazolidin-2-one
(4R)-3-(3-Methyl-1-oxobutyl)-4-(phenylMethyl)-2-oxazolidinone
Oxazolidinone, 3-(3-methyl-1-oxobutyl)-4-(phenylmethyl)-, (4R)-
3-(3-Methyl-1-oxobutyl)-4-(p henylMethyl)-, (R)-2-Oxazolidinone
物理化学性质
制备方法
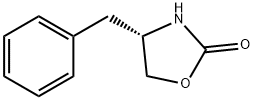
90719-32-7
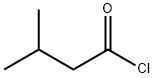
108-12-3

145589-03-3
一般步骤:在0℃下,将4-二甲基氨基吡啶(2.55g,21.3mmol)和三乙胺(46.9ml,340.8mmol)的二氯甲烷(100ml)溶液缓慢加入(S)-4-苄基-2-恶唑烷酮(37.7g,213mmol)的二氯甲烷(300ml)溶液中。随后,在保持内部温度低于10℃的条件下,逐滴加入异戊酰氯(33.75ml,207mmol)的二氯甲烷(50ml)溶液。反应混合物在10℃下搅拌30分钟后,过滤去除形成的盐。向滤液中加入水(100ml)进行相分离。有机相依次用水(100ml)和盐水(100ml)洗涤,经无水硫酸钠干燥后,减压浓缩得到53g黄色油状物,该产物在静置后固化为固体(产率95%)。产物经1H NMR(300MHz, CDCl3, 298K)和13C NMR(75MHz, CDCl3, 298K)表征,确认为目标化合物3-异戊酰基-4(R)-苄基-2-噁唑烷酮。
参考文献:
[1] Helvetica Chimica Acta, 2012, vol. 95, # 10, p. 1937 - 1945,9
[2] Patent: WO2011/151442, 2011, A2. Location in patent: Page/Page column 24
[3] Patent: US2013/71899, 2013, A1. Location in patent: Paragraph 0124-0127
[4] Journal of Medicinal Chemistry, 2016, vol. 59, # 23, p. 10530 - 10548
[5] Organic Process Research and Development, 2015, vol. 19, # 6, p. 611 - 617
