146679-66-5
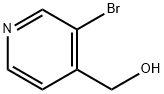 146679-66-5 结构式
146679-66-5 结构式
基本信息
3-溴-4-吡啶甲醇
(3-Bromopyridin-4-yl)
3-Bromo-4-pyridinemethanol
3-Bromopyridine-4-methanol
(3-Bromo-4-pyridyl)methanol
4-PyridineMethanol, 3-broMo-
(3-Bromo-4-pyridinyl)methanol
3-Bromo-4-(hydroxymethyl)pyridine
3-Bromopyridine-4-methanol ISO 9001:2015 REACH
物理化学性质
安全数据
制备方法
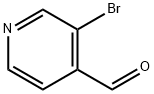
70201-43-3

146679-66-5
以3-溴吡啶-4-醛为原料合成3-溴吡啶-4-甲醇的一般步骤:在0℃条件下,向3-溴-4-吡啶甲醛(3.0g,16.2mmol)的无水甲醇(40mL)溶液中缓慢加入NaBH4(0.736g,19.5mmol)。在氮气保护下,将反应混合物于0℃持续搅拌2小时。反应完成后,通过减压蒸馏除去溶剂。向残余物中加入水和乙酸乙酯进行萃取,分离有机层后用蒸馏水洗涤,无水Na2SO4干燥,最后减压浓缩得到目标产物3-溴吡啶-4-甲醇(3.021g,收率100%),为白色粉末。产物结构经1H NMR(CDCl3, 300MHz, 298K, δ ppm):8.61(s, 1H),8.51(d, 1H, J = 4.8Hz),7.55(d, 1H, J = 4.8Hz),4.76(s, 2H),2.89(s, 1H);13C NMR(CDCl3, 75.5MHz, 298K, δ ppm):151.14, 149.45, 148.54, 122.47, 119.90, 63.47;GC/MS(m/z):188;IR(KBr, v, cm-1):3152, 2894, 2829, 1593, 1447, 1401, 1333, 1223, 1170, 1070, 1024, 834, 705, 599确认。
参考文献:
[1] Patent: EP2759536, 2014, A1. Location in patent: Paragraph 0067; 0068
[2] Patent: WO2014/114742, 2014, A1. Location in patent: Page/Page column 25
[3] Patent: WO2015/25172, 2015, A1. Location in patent: Page/Page column 90
[4] Journal of Medicinal Chemistry, 2015, vol. 58, # 20, p. 8309 - 8313
[5] Organic Letters, 2008, vol. 10, # 13, p. 2701 - 2704
